Article content
There’s a price shocker coming at the pumps.

|
|
International cannabis companies are showing interest in Brazil, both its large consumer market for medicinal products and a proposal that could legalize planting of the crop.
Major producers like Colombia’s Clever Leaves and Canada‘s Canopy Growth are developing and selling medicinal cannabis products to a Brazilian consumer segment estimated at 10 million to 13 million people. This results from a 2019 regulatory change allowing the import, sale and manufacturing of such products.
But permission for cultivation of hemp and cannabis in Brazil would be a bigger prize. If granted, the industry could blossom in four to five years, based on the experience of other countries such as Colombia.
“By 2025, I would like to be planting hemp in the interior of Pernambuco,” said José Bacellar, founder of Canada‘s VerdeMed, referring to a northeastern state known for illegal marijuana growing.
A proposal that would legalize cultivation was approved in June by a congressional committee. Lawmakers are weighing if it could be fast-tracked to the Senate for approval. If passed there, President Jair Bolsonaro would have to sign it into law.
While Bolsonaro’s far-right positions may seem an unlikely match for the bill, the proposal has support from some members of the powerful farm sector, a key constituency that helped him win the 2018 election.
SILICON VALLEY OF CANNABIS
In the quiet town of Viçosa in southeastern Brazil – which some call the Silicon Valley of cannabis – researchers are developing a hemp variety better suited to the tropics.
If the law is changed and research is successful, Brazil could become a top grower of cannabis and hemp, experts said.
Sérgio Rocha, director of ag-tech startup Adwa which is developing the hemp strain for Brazil, said about 3 million square kilometers (1.2 million square miles) of land would potentially be suitable for cultivating the new variety.
Brazil could overtake China, the world’s largest hemp producer, which has about 670 square kilometers (259 square miles) planted.
“Using a part of Brazil’s agricultural land would be enough to give the country the title of world’s largest producer and exporter of hemp fibers, seeds and flowers for medicinal and industrial purposes,” said Dennys Zsolt, an agronomist specializing in the plant.
Brazil bans growing of Cannabis sativa L, the plant that produces hemp and marijuana. Hemp, which has less than 0.3% of the psychoactive compound THC, contains CBD or cannabidiol. This non-intoxicating ingredient has been touted as beneficial for many health conditions including childhood epilepsy.
Growing the plants in Brazil would lay the foundation for a vertically integrated industry. A stable source of the raw material would support manufacturing of medicinal cannabis products, growth of a retail market and exports. Recreational cannabis would remain illegal.
Gabriela Cezar, chief executive of New York-based Panarea Partners investment banking firm, sees Brazil playing a leading role in hemp in Latin America, a region she calls the “epicenter of world hemp production.”
Panarea plans to form a Brazilian cannabis company focused on pharmaceutical products for pets while seeking to broker more cannabis deals in Brazil.
TROPICAL ADVANTAGE
Among Brazil’s advantages are lower growing costs because its warm climate allows plants to grow outdoors compared to greenhouses in some countries. Stable hours of sunlight due to Brazil’s proximity to the equator are another plus.
Canopy Growth is “actively monitoring the advancement of hemp regulations in Brazil,” David Culver, the company’s vice president of global government relations, said.
But nothing is certain without the change to Brazil’s law, though some signs suggest the prospects are favorable. When Rocha spoke to a congressional committee about hemp in 2019, he was surprised that conservative lawmakers were not hostile.
“After I finished presenting the maps and hemp’s potential, I was applauded,” he said.
Although the farm caucus has not taken a formal position, members of the group said a majority in both houses of Congress back the proposal. The farm caucus controls slightly fewer than half the seats in the two chambers, and the law requires approval by a simple majority.
Center-right lawmaker Fausto Pinato, a member of the farm caucus, said he supports the bill. “If you are authorizing the sale, why not cultivation?,” he said.
(Reporting by Ana Mano in São Paulo, Jimin Kang in Seul and Maximilian Heath in Buenos Aires; Editing by Cynthia Osterman)
Gas prices have not been this high since August 2022


There’s a price shocker coming at the pumps.
Advertisement 2
Article content
Gas in Ontario, including the GTA, will go up 14 cents a litre overnight for customers filling up on Thursday, says Dan McTeague, the president of Canadians for Affordable Energy.
Article content
“So going from $1.65.9 (per litre) going to $1.79.9,” said McTeague adding the increase will affect the entire province except for northwestern Ontario, which gets its prices from the prairies market.
“That’s the highest level since August, 2022, almost two years ago,” he added.
Recommended from Editorial
McTeague said the reason for the price hike is that stations are switching over to summer-blend gasoline.
“Around this time of year prices go up to reflect the new blend of gasoline, which is more expensive to make,” he explained. “Butane is used in the winter, for gasoline, whereas in the summer it’s alkyaltes. Alkyaltes are extremely expensive.”
Advertisement 3
Article content
“In the winter you want your ignition to start quickly in cold temperatures, you uses volatile butane. You take that out in the summer. That’s a big difference. This is going to be around for awhile and it could get higher,” McTeague said.
McTeague also blamed the rise in gas prices in Canada on the carbon tax increase, the rising price of oil, and the weak Canadian dollar.
“It just makes a bad situation worse,” he said. “It’s just another brick in the wall, another load on the camel’s bank. The cost of denying our resources, blocking pipelines, is one of the most significant reasons why the Canadian dollar is so weak.”
Article content

CALGARY — A wildfire in west-central Alberta that was sparked by a natural gas pipeline rupture is under control, but an investigation into what caused the pipeline to break could take months or even years.
As of Wednesday morning, there was very little fire activity left in Yellowhead County, where a 10-hectare fire burned on Tuesday about 40 kilometres northwest of Edson.
“But for it to be considered extinguished, we’re going to have to hot spot,” said Caroline Charbonneau, area information co-ordinator with Alberta Forestry and Parks.
“That means we’ll have to dig into the ground, look and feel for hot spots, and then douse it with water. And that could take several days.”
ADVERTISEMENT
The fire on Tuesday, which occurred as much of Alberta is dealing with extremely dry early spring conditions, was sparked when a natural gas pipeline owned by TC Energy Corp. ruptured.
There were no injuries, and the fire was never a threat to any surrounding communities. The affected pipeline segment was isolated and shut in and there is no more gas leaking from the pipeline.
The Canada Energy Regulator had inspectors on site Wednesday to monitor the company’s response and the Transportation Safety Board is investigating the incident.
According to CER, there have been 12 natural gas pipeline ruptures in Canada since 2008, and Tuesday’s incident near Edson was the first rupture on that particular pipeline within that time period.
The 36-inch diameter pipe that ruptured is part of TC Energy’s NGTL pipeline system, which transports natural gas from Alberta and northeast B.C. to domestic and export markets. The system spans 24,631 kilometres and connects with TC Energy’s Canadian Mainline system, Foothills system and other third-party pipelines.
The NGTL pipeline system is like a web made up of different lines that have been developed in stages.
In 2022, there was a rupture on a separate part of the system that resulted in an explosion and fire near Fox Creek, Alta. There were no injuries.
A TSB investigation into that incident took more than 14 months, and concluded that the pipeline ruptured due to reduced pipe wall strength caused by external corrosion.
While the primary risk of a crude oil pipeline leak is an oil spill that harms the local ecosystem, natural gas pipeline ruptures can and do result in fires or explosions, said Bill Caram, executive director of the Pipeline Safety Trust, a U.S.-based non-profit organization.
“The chances are extremely high that a molecule of natural gas that enters a pipeline will go through that pipeline without a failure. Pipelines are quite safe, and when you look at incident rates compared to other modes of transportation like rail or truck, they are much less likely to have a failure,” Caram said.
“But what you don’t get a sense of by looking at the risks of pipelines in that way is how catastrophic a failure can be when it does happen.”
According to the TSB, there were 19 recorded incidences of fires related to pipelines in Canada between 2012 and 2022.
The TSB’s most recent report on pipeline transportation safety in Canada states that in 2022 there were 100 companies transporting either oil or gas or both in the federally regulated pipeline system, which includes approximately 19,950 km of oil pipelines and approximately 48,700 km of natural gas pipelines.
That year, there were 67 pipeline transportation accidents and incidents on federally regulated pipeline systems, according to the report.
That number was well below the 10-year average of 112 occurrences, and was also the lowest number of occurrences since 2019, when 52 pipeline accidents or incidents were recorded by the TSB.
The TSB defines a pipeline “accident” as an incident that results in a person being injured or killed, a fire or explosion, or significant damage to the pipeline affecting its operation.
Less severe pipeline events that involve the uncontrolled release of a commodity or a precautionary or emergency shutdown are classified by the TSB as “incidents.”
There have been no fatal accidents directly resulting from the operation of a federally regulated pipeline system since the inception of the TSB in 1990.
This report by The Canadian Press was first published April 17, 2024.
Companies in this story: (TSX:TRP)
Amanda Stephenson, The Canadian Press
Police say one former and one current employee of Air Canada are among the nine suspects that are facing charges in connection with the gold heist at Pearson International Airport last year.
At a news conference Wednesday on the one-year anniversary of the heist, police confirmed that five suspects were arrested and four others are facing charges in connection with the largest gold theft in Canadian history.
Police said the suspects face a total of 19 charges and Canada-wide warrants have been issued for the arrest of three of the suspects who have not yet been apprehended. All of the suspects arrested in connection with the heist have been released on bail, police confirmed in a news release issued Wednesday.
Peel Regional Police Chief Nishan Duraiappah said the heist was “carefully planned” by a “well-organized group of criminals.”
“This story is a sensational one and one which probably, we jokingly say, belongs in a Netflix series,” he said.
Police said 6,600 gold bars were stolen from Air Canada’s cargo facility on the evening of April 17, 2023 by a suspect who arrived at the warehouse in a five-tonne delivery truck.
The gold, along with about $2.5 million in foreign currency, had been shipped to Toronto from Zurich in the hull of an Air Canada plane and was offloaded to an Air Canada cargo facility shortly after the flight landed at Pearson Airport that afternoon.
Police allege that the suspect came into possession of the stolen gold and bank notes after presenting Air Canada personnel with a fraudulent airway bill.
“The airway bill was for a legitimate shipment of seafood that was picked up the day before,” Det.-Sgt. Mike Mavity, the major case manager for the joint investigation, dubbed Project 24K, told reporters on Wednesday.
“This duplicate airway bill was printed off from a printer within Air Canada cargo.”


Brinks Canada, which was hired to provide security and logistics services for the transportation of the shipment, showed up at the facility a few hours later to pick up the items, police said.
According to investigators, when Air Canada employees tried to locate the container, they realized it was missing and quickly launched an internal investigation. Police were notified about the stolen goods shortly before 3 a.m. the following day, Mavity said.
An exhaustive investigation followed, police said, with officers reviewing video surveillance footage from 225 businesses and residences in an effort to track the path of the truck, which has since been recovered.
Mavity said that last summer, they identified 25-year-old Durante King-McLean as the driver of the truck but were unable to locate him.
In September 2023, Mavity said King-McLean was stopped in rental vehicle by Pennsylvania State Police near Chambersburg, Pennsylvania.
“After a brief foot chase, he was detained and troopers located 65 illegal firearms in the vehicle,” Mavity said Wednesday.
According to Mavity, investigators believe that the stolen gold was melted down and sold and the proceeds were used to purchase illegal guns for a firearms trafficking operation.
He said members of Project 24K have been liaising with the U.S. Alcohol, Tobacco, and Firearms Bureau (ATF) with respect to this aspect of the investigation.
Speaking at the news conference on Wednesday, a representative from the ATF said the law enforcement agency believes the 65 guns seized during the arrest of King-McLean were bound for Canada.
While King-McLean is currently in custody in the United States, he is now wanted on multiple charges in connection with the gold theft.
“We are alleging that some individuals who participated in this gold theft are also involved in aspects of this firearms trafficking,” Mavity added.


Two “debt lists” were found by investigators at separate locations during the investigation, police said.
“A common term in drug trafficking investigations, we believe these lists actually show where the money was distributed when the gold was sold by the suspects,” Mavity said.
He said the names on both lists are “consistent” and police are trying to identify all of those identified.


Police said one current Air Canada employee, identified as 54-year-old Brampton resident Parmpal Sidhu, has been charged with theft over $5,000 and conspiracy to commit an indictable offence. A Canada-wide warrant has been issued 31-year-old Simran Preet Panesar, who police said resigned from his position as a manager at Air Canada back in the summer.
“He has been known to us since early on in the investigation. He actually led a tour for Peel Regional Police before we knew his involvement,” Mavity said Wednesday.
He added that police have an idea where Panesar may be but did not elaborate on a possible location.
Mavity said he believes the suspects needed employees on the inside to carry out the heist.
“Because of their position within Air Canada, in my opinion, yeah they needed people inside Air Canada to facilitate this theft,” he said.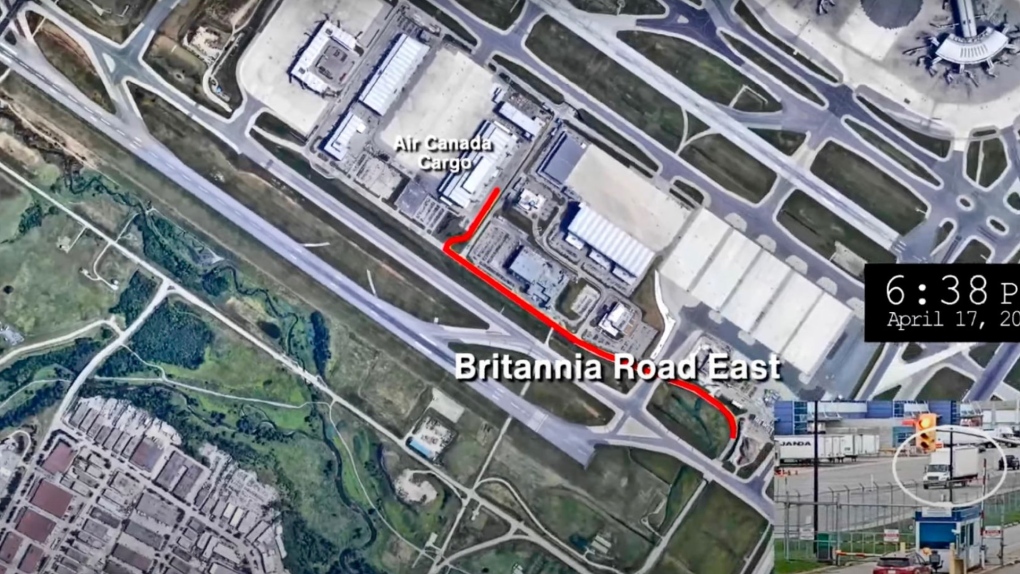

Firefighters battle wildfire near Edson, Alta., after natural gas line rupture – CBC.ca




iPhone 15 Pro Desperado Mafia model launched at over ₹6.5 lakh- All details about this luxury iPhone from Caviar – HT Tech




Lululemon unveils Canada's official Olympic kit for the Paris games – National Post




Trump gave MAGA politicians permission to move left on abortion. Some are taking it. – Semafor




Anti-Trump Republican Larry Hogan navigates dangerous political terrain in pivotal Senate contest – Toronto Star




Astronomers discover Milky Way's heaviest known black hole – Xinhua
'Terrific news': Chief economist reacts to latest inflation data – BNN Bloomberg
Traders Place Bets On $250 Oil – OilPrice.com
Comments