Health
COVID-19 vaccine development reported in various countries – Macau Business

Many countries have recently reported development in their COVID-19 vaccine trials, bringing hope to the world amid the still raging pandemic, while health experts called for an objective, rational and scientific attitude towards vaccine candidates.
At least 24 COVID-19 candidate vaccines are in clinical evaluation, and another 142 in preclinical evaluation, according to data released by the World Health Organization (WHO) on Monday.
On Monday alone, four countries reported their new research results of COVID-19 vaccines.
Chinese experts said in a new study published in medical journal The Lancet that a phase 2 trial of a COVID-19 vaccine candidate has found that the vaccine is safe and induces an immune response.
“The phase 2 trial adds further evidence on safety and immunogenicity in a larger population than the phase 1 trial. This is an important step in evaluating this early-stage experimental vaccine and phase 3 trials are now underway,” said Professor Fengcai Zhu from Jiangsu Provincial Center for Disease Control and Prevention, China.
A report published also in The Lancet reveals the results of the phase 1/2 trial of the Oxford coronavirus vaccine ChAdOx1 nCoV-19. It indicates no early safety concerns and produces strong immune response.
According to the University of Oxford, the trial involves more than 1,000 healthy adult volunteers. The vaccine provoked a T cell response (white blood cells that can attack cells infected with the SARS-CoV-2 virus) within 14 days of vaccination, and an antibody response within 28 days.
The Russian Defense Ministry said that together with the Gamaleya Scientific Research Institute of Epidemiology and Microbiology, it has successfully completed clinical trials of COVID-19 vaccine with the participation of volunteers.
“Without exception, all volunteers, having received immunity from the coronavirus, felt fine,” First Deputy Defense Minister of the Russian Federation Ruslan Khadzhismelovich Tsaikov told Moscow’s Argumenty i Fakty newspaper.
German biotech company BioNTech and U.S. pharmaceutical corporation Pfizer announced that data from their experimental COVID-19 vaccine showed that it was safe, and induced an immune response and high-level T-cell responses against the novel coronavirus in patients.
Earlier this month, research institutes in other countries also reported progress in their COVID-19 vaccine trials.
In a study published July 14 in the New England Journal of Medicine, researchers reported that the COVID-19 vaccine mRNA-1273, co-developed by the U.S. National Institute of Allergy and Infectious Diseases and U.S. biotech firm Moderna Therapeutics, induced immune responses and no serious side effects in volunteers in the second clinical trial.
Moreover, the University of Queensland in Australia, Chulalongkorn University in Thailand, among other institutes, also reported positive results in their COVID-19 vaccine research.
Despite the good news on COVID-19 candidate vaccines, experts warned of uncertainties of vaccine development and clinical trials, as well as multiple risks and challenges including virus mutation, while appealing for an objective, rational and scientific attitude towards vaccine research.
Dr. Michael Ryan, executive director of the WHO Health Emergencies Program, said on July 3 that there is no accurate timetable for the delivery of COVID-19 vaccines.
Although the preliminary data of some candidate vaccines is quite promising, it is still unpredictable which one is totally clinically effective, he told a briefing.
While a vaccine candidate might show its effectiveness by year’s end, the question is how soon it could be mass produced, he added.
Saudi Arabia’s Asharq al-Awsat newspaper said recently that researchers should avoid being over-optimistic about the significant progress of some candidate vaccines in clinical trials, and wait for further trial results.
Merck CEO Kenneth Frazier said that the effectiveness of developing vaccines cannot be guaranteed.
Some studies showed that the level of anti-bodies will decline after COVID-19 patients recover. Joint efforts of scientific research teams are needed to tackle issues including how COVID-19 vaccines provide sufficient long-term immunity and how to deal with possible virus variation.
The WHO urged that before a COVID-19 vaccine is officially available, all countries should consistently take full-flung prevention and control measures.
Health
Canada's opioid deaths double in 2 years, men in their 20s, 30s hit hardest – Surrey Now Leader

Opioid-related deaths doubled in Canada between 2019 and the end of 2021, with Manitoba, Saskatchewan and Alberta experiencing a dramatic jump, mostly among men in their 20s and 30s, says a new study that calls for targeted harm-reduction policies.
Researchers from the University of Toronto analyzed accidental opioid-related deaths between Jan. 1, 2019 and Dec. 31, 2021 in those provinces as well as British Columbia, Ontario, Quebec, New Brunswick and Nova Scotia, and the Northwest Territories.
Manitoba saw the sharpest rise in overdose deaths for those aged 30 to 39 – reaching 500 deaths per million population, more than five times the 89 deaths per million population recorded at the beginning of the study period.
In Saskatchewan, the death toll for that age group nearly tripled to 424 per million, up from 146 per million, while Alberta’s rate spiked more than 2.5 times to 729 fatalities per million, up from 272 per million. Ontario’s death rate reached 384, up from 210 per million.
British Columbia, which has been the epicentre of the overdose crisis, recorded 229 deaths per million for that age group in 2019, climbing to 394 in 2020. All data for 2021 from that province’s coroners service was not yet available when researchers completed their work based on information collected by the Public Health Agency of Canada.
Nationally, the annual number of opioid overdose deaths surged from 3,007 to 6,222 over the three-year study period, which researchers note coincided with pandemic public health measures that reduced access to harm reduction programs and imposed border restrictions that may have increased the toxicity of the drug supply.
“In addition, for many, the pandemic exacerbated feelings of anxiety, uncertainty, and loneliness, contributing to increased substance use globally,” they said.
The study was published Monday in the Canadian Medical Association Journal.
Senior author Tara Gomes said one in four deaths involved people in their 20s and 30s. More than 70 per cent of the overall deaths were among men.
A spokesman with the coroners service in British Columbia said 78 per cent of people that fatally overdosed in that province between 2019 and the end of 2021 were men.
The sharp surge in fatal overdoses – especially among young adults in the Prairies – suggests provinces must act quickly, said Gomes, an epidemiologist who called for more harm-reduction services including supervised consumption sites.
“Being slow and not being as nimble as we would like to be in our responses can have really devastating impacts,” said Gomes, also lead principal investigator of the Ontario Drug Policy Research Network.
Bernadette Smith, Manitoba’s minister of housing, addiction, homelessness and mental health, said the province plans to open its first supervised consumption site in Winnipeg next year and will also offer drug-testing machines so people can check if their illicit substances are toxic.
“We came out of a previous government that didn’t take a harm-reduction approach, unfortunately,” said the New Democrat, whose party defeated the Progressive Conservatives last fall.
“We’re working with front-line organizations because they have not been listened to or worked with for the last seven years in our province, which has been a real problem.”
Manitoba plans to train family doctors to treat addiction with medications including Suboxone and methadone, said Smith, noting the physicians typically refer patients to detox for care.
“We’re creating a model so that folks aren’t having to go to a bunch of different places to get different services,” said Smith.
She declined to say whether Manitobans will have access to a prescribed safer supply of drugs.
Tanya Hornbuckle of Edmonton said her son Joel Wolstenholme was 30 when he died in 2022. He became addicted to illicit substances at about age 14, starting with cannabis before shifting to methamphetamine, cocaine and other drugs that were increasingly laced with fentanyl.
He also battled a mental illness but getting help for both that issue and addiction in a single service was challenging, Hornbuckle said.
Wolstenholme tried multiple times to detox but there were never enough beds at a clinic where people had to line up at 8 a.m., she said.
“It would happen over and over and then he would call me. I went and stood in line or I drove him there and waited with him in the lineup. They wouldn’t have enough beds.”
Her son’s anxieties and addiction worsened when pandemic restrictions prevented her from entering an emergency room with him because he did not trust staff, Hornbuckle said.
On Feb. 6, 2022, Hornbuckle went to her son’s home so they could cook together. She found him dead.
The Alberta government’s strategy of focusing more on recovery and abstinence-based treatment than harm reduction, mental health and housing is the wrong approach, said Hornbuckle, noting that for a time her son slept in parks and abandoned houses after losing his vehicle and apartment to addiction.
Rebecca Haines-Saah, an associate professor of community health services at the University of Calgary, called the deaths of young people from overdose a tragedy, and said many more suffer from brain injury due to toxic substances.
“Obviously, we have the incorrect response. We do not have the approach and services available to keep people alive,” said Haines-Saah, who also called for more harm-reduction services.
“We don’t have a full-scale public health response that is required. We don’t have any plans to fund anything that relates to what we would call harm reduction.”
Much of the current approach to addiction excludes a large number of recreational drug users, said Gomes. She said between a third and half of the deaths in Ontario involved people without an opioid use disorder diagnosis.
“So, focusing on (residential treatment) alone is something that really concerns me because we really need to make sure that we have different options for different people.”
READ ALSO: Stories from the overdose crisis’ front lines
READ ALSO: Make overdose education mandatory in B.C. schools amid drug emergency, advocates say
Health
Manitoba significantly impacted by opioid-related deaths at start of pandemic | CTV News – CTV News Winnipeg

A new study out of Ontario and posted in the Canadian Medical Association Journal is highlighting the significant increase in accidental opioid-related deaths in Canada leading into the COVID-19 pandemic, with Manitoba being one of the most impacted provinces in the country.
The research looked at opioid-related deaths between 2019 and 2021 in nine provinces and territories in Canada.
Across Canada, opioid-related deaths more than doubled from 2019 with 3,007, to 6,222 in 2021.
It also found the years of life lost per 100,000 people climbed from 3.5 years in 2019 to seven in 2021.
After dipping halfway through 2019, opioid-related deaths spiked dramatically through the first quarter of 2020 and spiked again in the third quarter of 2021.
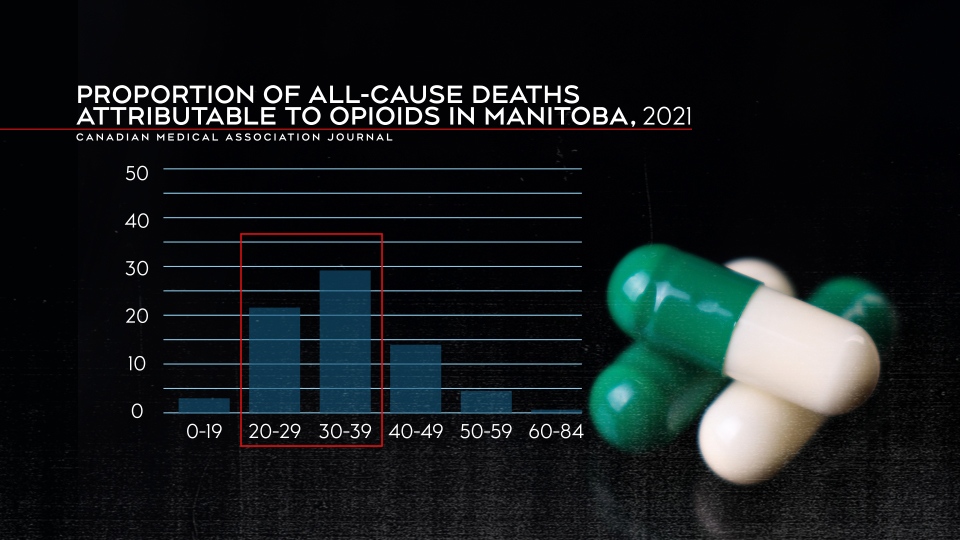
People in their 20s and 30s were most impacted by opioid deaths as they represented 29.3 per cent of all deaths in people aged 20 to 29 and 29 per cent of all deaths for people between 30 and 39.
“The disproportionate loss of life in this demographic group highlights the critical need for targeted prevention efforts,” the report said.
The data also showed men were much more likely to suffer an opioid-related death compared to women, with more than 4,500 deaths in 2021 compared to more than 1,600 women.
Manitoba one of the most impacted provinces by opioid-related deaths
Breaking down the provinces individually, the research found the Prairie provinces were impacted the most by opioid-related deaths.
Alberta and Saskatchewan both recorded fatality numbers that more than doubled between 2019 and 2021 – 619 deaths to 1,618 in Alberta and 109 to 322 deaths in Saskatchewan.
Meanwhile, Manitoba’s opioid-related deaths spiked nearly five-fold by 2021. There were 54 deaths in the province in 2019 and by the end of 2021, there were 263.
“In Manitoba, 70 per cent of opioid toxicity deaths in 2019 had fentanyl or fentanyl analogues detected, increasing to 86 per cent in 2020,” the report said.
Arlene Last-Kolb, a member of Moms Stop the Harm, lost her son Jessie to fentanyl drug poisoning in 2014.
She said the toxic drug supply is one of the main issues that needs to be addressed.
“We’re losing a whole generation of young people like my son,” Last-Kolb said. “It’s going to take a lot more than safe spaces and more treatment to address the toxic drug supply, including opiates, fentanyl that we have on our streets.”
Proportion of all-cause deaths attributable to opioids in Manitoba in 2021. (Canadian Medical Association Journal)
The years of life lost also jumped dramatically in Manitoba, going from 1.8 per 100,000 to 8.5 per 100,000 in 2021.
Those in the 30 to 39 age range were most impacted by opioid-related deaths in Manitoba. Almost 30 per cent of deaths in that age group were attributable to opioids.
Marion Willis, the founder and executive director of St. Boniface Street Links, called the numbers horrifying. She says something needs to be done as soon as possible.
“If that is not the strongest statement ever to support that we need a plan to address the drug crisis in this city, in this province – I don’t know what it takes,” said Willis.
She said plans for a new safe consumption site are a good first step, but agreed the drug supply also needs to be addressed.
“Safe consumption needs to include safer supply, or will we still have people using the same toxic drugs off the street.”
Bernadette Smith, the minister of housing, addictions and homelessness, said the province has a number of items on its agenda to help deal with the problem.
“That’s exactly what our government is doing. So supervised consumption site, drug testing machines, that’s our first step – getting those up and running,” said Smith.
However, Willis and Last-Kolb want to see action now.
“This is a challenge that is impacting all members of our human family. We’re all losing our loved ones, you know, from the wealthiest families to the poorest families. This is affecting everybody,” said Willis.
“It’s frustrating to talk about things that are going to happen down the road when somebody dies here every single day,” said Last-Kolb.
Health
Dr. Theresa Tam to visit 3 Nunavut communities regarding TB outbreaks – pentictonherald.ca


Canada’s chief public health officer, Dr. Theresa Tam, will tour Nunavut this week.
The communities of Naujaat, Pond Inlet (Mittimatalik), and Iqaluit are on her itinerary, to coincide with the launch of a community-wide screening clinic for tuberculosis (TB) in Naujaat.
Naujaat and Pond Inlet are currently experiencing TB outbreaks. Tam’s visit to Nunavut will be an opportunity for Canada’s top doctor to work with Nunavut Tunngavik Incorporated (NTI) and the Government of Nunavut, to observe social issues, such as housing shortages and food insecurity, that have significant impacts on the health of Nunavut Inuit, according to NTI.
Tam will be accompanied in her travels by NTI vice-president Paul Irngaut, Government of Nunavut Minister of Health John Main, and Nunavut’s deputy chief public health officer Dr. Ekua Agyemang, among others.
TNCMS.AdManager.init
(
domain: “www.pentictonherald.ca”,
secureDomain: “pentictonherald.ca”,
virtualregion:
popup: __tnt.ads.popup
,
callback:
expandable: __tnt.ads.expandable,
html: __tnt.ads.html,
image: __tnt.ads.image,
pagecurl: __tnt.ads.curl,
text: __tnt.ads.text,
video: __tnt.ads.video
);
“Nunavut Inuit face challenges that don’t affect most Canadians when accessing healthcare,” Irngaut said. “Having Dr. Tam on the ground visiting Nunavut communities will give her the opportunity to see firsthand some of the barriers that Inuit face when trying to navigate the healthcare system in Nunavut.”
Canada’s top doctor’s tour will conclude with two days of meetings and events in Iqaluit.
Although TB remains the focus of the visit for Tam, she will also meet with community groups and organizations to discuss related territorial issues such as homelessness, health education, mental health and health research initiatives.
-
Media19 hours ago
DJT Stock Plunges After Trump Media Files to Issue Shares
-
Business18 hours ago
FFAW, ASP Pleased With Resumption of Crab Fishery – VOCM
-
Media18 hours ago
Marjorie Taylor Greene won’t say what happened to her Trump Media stock
-
Business19 hours ago
Javier Blas 10 Things Oil Traders Need to Know About Iran's Attack on Israel – OilPrice.com
-
Media17 hours ago
Trump Media stock slides again to bring it nearly 60% below its peak as euphoria fades – National Post
-



 Politics18 hours ago
Politics18 hours agoIn cutting out politics, A24 movie 'Civil War' fails viewers – Los Angeles Times
-
Business24 hours ago
A government mortgage policy that makes sense – with one glaring question – The Globe and Mail
-
Art20 hours ago
Pinot & Pottery Bubble Art – Castanet.net




