Tissue-specific telomerase expression rescues gut aging
To investigate how telomere-dependent gut aging impacts the organism, we generated a zebrafish transgenic line harboring a Cre-inducible zebrafish tert transgene driven by an enterocyte-specific fabp2 promoter23 in a tert+/− genetic background (Fig. 1a). After crossing this line with tert+/− fish, we induced the tert transgene expression by microinjection of Cre mRNA in one-cell-stage embryos, creating the following sibling fish: (1) tert−/− containing the full construct (tert−/− no Cre); (2) tert−/−-expressing tert transgene (tert−/− + Cre); and (3) tert+/+ containing the full construct (wild-type (WT)).
As expected, we did not detect expression of the tert transgene in mock-injected fish, while Cre microinjection resulted in the excision of the STOP cassette and tert transgene expression (Fig. 1b, left). This led to an approximate fivefold enrichment of total tert mRNA (endogenous and transgene tert mRNA) in the gut of tert−/− + Cre fish when compared to mock-injected control tissues (tert−/− no Cre and WT; Fig. 1b, right). Consequently, we observed a higher telomerase activity in tert−/− + Cre compared to both WT and tert−/− no Cre (Extended Data Fig. 1f). To test whether expression of the tert transgene is sufficient to prevent telomere shortening, we performed telomere restriction fragment (TRF) analysis on gut samples of 9-month-old fish. As described previously14,15, we noted that the range of telomere length in the gut of WT fish exhibited a bimodal pattern (Extended Data Fig. 1c,d). This pattern reflects the differences in telomere length between cell types. The telomere length of WT blood cells was longer (approximately 19 kb) than other tissues (approximately 9 kb) leading to a two-peak densitometry pattern14,15,24. Reflecting the requirement of telomerase to sustain long telomeres in blood cells, the telomere length of tert−/− blood cells was drastically reduced compared to WT (as seen by the loss of the longer peak; Extended Data Fig. 1d)14,15. Even though expression of tert complementary DNA (cDNA) driven by the fabp2 promoter did not restore telomere length to WT levels, induction of the tert transgene was sufficient to elongate telomeres in the whole-gut tissues of tert−/− + Cre fish (7.9–8.4 kb, n = 6–7, P < 0.05; Fig. 1c and Extended Data Fig. 1c,e). Like tert−/− no Cre fish, tert−/− + Cre fish lacked the longer telomere peak, indicating that the tert transgene is not expressed in blood cells. As described previously14,15, telomere shortening in the gut of tert−/− no Cre fish leads to an increase in DNA damage, as observed by γH2AX immunofluorescence and p53 protein levels, when compared to WT fish (Fig. 1d,e). Consistent with telomere elongation, these markers are reverted by telomerase expression in the gut of tert−/− + Cre fish. Thus, tert transgene expression is sufficient to counteract telomere dysfunction in the gut of tert−/− fish by extending telomere length.
To test whether tert transgene expression can rescue the aging defects of telomerase-deficient animals, we analyzed the gut of 9-month-old fish. As observed previously14,15, the gut of tert−/− no Cre fish showed reduced cell proliferation compared to WT fish. Enterocyte-specific telomerase expression rescued the proliferative capacity of this organ to WT levels (Fig. 1f). Senescence-associated β-galactosidase (SA-β-gal) assays and transcription levels of the senescence-associated genes ink4a/b (p15/16) and cdkn1a (p21) revealed that telomerase expression reduced cell senescence to WT levels (Fig. 1g–i). Consistent with our previous work17, we detected no differences in apoptosis in the gut of WT, tert−/− no Cre and tert−/− + Cre of 9-month-old fish (Extended Data Fig. 2a).
These cellular defects observed in tert−/− fish impact tissue integrity14,15,17. We observed that tert−/− no Cre fish exhibited morphological tissue defects with thickening of the lamina propria (Fig. 1j,k). Loss of intestinal barrier integrity led to activation of the Yes-associated protein (YAP) transcription factor responsible for tissue regeneration25,26. Consistent with loss of gut integrity, expression of the YAP target genes cyr61 and ctgf was increased in tert−/− no Cre fish (Fig. 1l,m). Likewise, claudin-2 mRNA levels were higher in tert−/− no Cre fish (Fig. 1n). Increased gene expression of the tight junction protein claudin-2 occurs during primate aging and enhances in vivo intestinal permeability27,28. Strikingly, all these phenotypes were rescued in tert−/− + Cre fish (Fig. 1j–n).
We observed that the number of proliferative cells in individual intervilli was negatively correlated with the thickness of the lamina propria (Extended Data Fig. 3). Plotting either individual intervilli (Extended Data Fig. 3a) or individual fish (Extended Data Fig. 3b), we noticed that WT and tert−/− + Cre clustered separately from tert−/− no Cre samples. In addition, we observed higher infiltration of total immune cells and neutrophils in the intestinal epithelium of the tert−/− no Cre fish compared to WT (Fig. 1o,p). In line with a rescue of intestinal integrity, the number of immune cells was reverted to WT levels in tert−/− + Cre fish. Considering that thickening of the gut lamina propria results from immune cell infiltrates, these results suggest that cell proliferation is locally affected by inflammation. Thus, rescuing tissue integrity promotes the proliferative capacity of the gut in part by reducing tissue inflammation.
Local effects
Gut tert rescues gene expression and metabolism
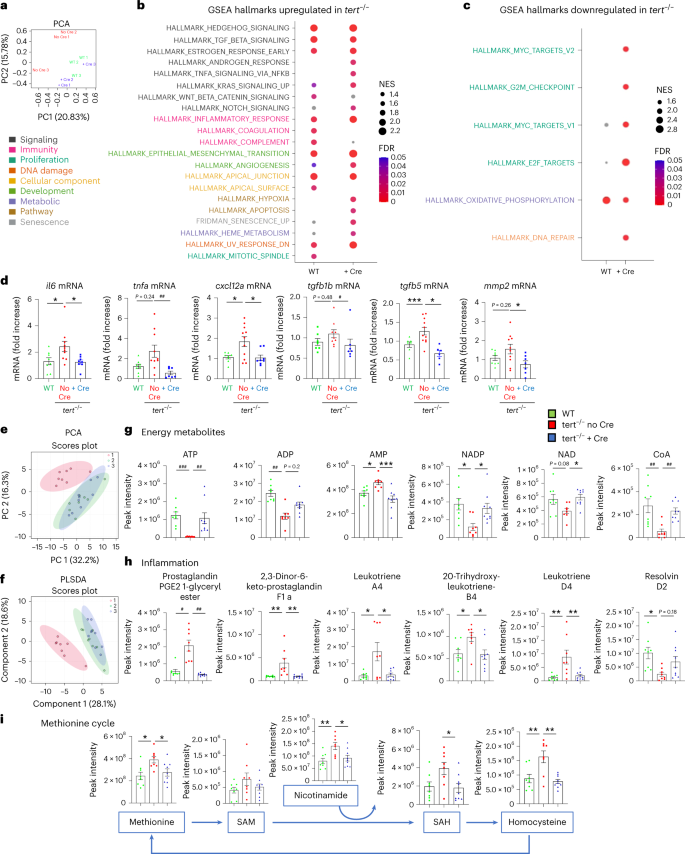
By comparing the expression profiles of whole-gut tissues using RNA sequencing (RNA-seq), we observed a distinguishable transcriptomics signature in tert−/− no Cre, while WT and tert−/− + Cre clustered together (Fig. 2a and Supplementary Data). Gene set enrichment analysis (GSEA) showed that most of the hallmarks were similarly deregulated in tert−/− no Cre than either WT or tert−/− + Cre. The transcriptomics profiles of the tert−/− no Cre gut are enriched in gene expression related to senescence, inflammation and morphogenesis (Fig. 2b), while the hallmarks of proliferation or oxidative phosphorylation are downregulated (Fig. 2c). We further validated this transcriptomics recovery of senescence-associated secretory phenotype (SASP)/inflammation-related genes by analyzing the transcription levels of the il6, tnfa, cxcl12a, tgfb1b, tgfb5 and mmp2 genes (Fig. 2d). In line with the previous results, these transcription profiles confirmed that telomerase expression rescued cell proliferation, loss of tissue integrity, senescence and inflammation seen in the gut of tert−/− no Cre fish.
Changes in metabolism have been associated with aging and might reflect cellular defects, such as gradual mitochondrial dysfunction with age29,30. Consistently, we previously showed that 9-month-old tert−/− gut exhibits mitochondrial dysfunction accompanied by low ATP and high reactive oxygen species (ROS) levels17. Unsupervised and supervised clustering analyses of metabolomics profiles revealed that both WT and tert−/− + Cre samples clustered tightly while tert−/− no Cre samples differed from the other groups (Fig. 2e,f and Extended Data Fig. 5a). Most metabolites were reduced (621) or enriched (141) in both WT and tert−/− + Cre when compared to tert−/− no Cre fish (Extended Data Fig. 5b). Consistent with our previous work17, we observed a drastic reduction of energetic metabolites, such as ATP, ADP, nicotinamide adenine dinucleotide (NAD), NAD phosphate (NADP) and coenzyme A (CoA), in tert−/− no Cre fish (Fig. 2g). Following the anaerobic glycolysis pathway, we noticed lower levels of glucose-6-phosphate and fructose 1,6-bisphosphate and higher amounts of pyruvate and lactate (Extended Data Fig. 5c). Considering that glucose did not vary between groups, our results suggest that the gut of tert−/− no Cre fish acquired higher levels of anaerobic glycolysis. We also detected higher pentose shunt activity in tert−/− no Cre gut, evidenced by increased amounts of ribose-5-phosphate and erythrose 4-phosphate (Extended Data Fig. 5d). Except for citrate levels, all the detected metabolites of the citric acid cycle were elevated in tert−/− no Cre fish (Extended Data Fig. 6a). Altogether, the gut energetic metabolism of tert−/− no Cre fish were engaged in uncoupled oxidative phosphorylation, consistent with damaged mitochondria, low ATP levels and higher production of ROS. By expressing tert transgene in the gut, tert−/− no Cre metabolic alterations were prevented in the entire tissue.
In line with our previous results depicting higher inflammation of tert−/− no Cre fish, we observed an overall increase in arachidonic metabolism with higher levels of pro-inflammatory molecules, such as prostaglandins and leukotrienes (Fig. 2h). Consistently, we detected lower amounts of anti-inflammatory resolvin D2 in tert−/− no Cre fish when compared to the other groups. Among the detected amino acids, methionine was significantly enriched in tert−/− no Cre gut compared to the other genotypes (Fig. 2i). We also observed an overall increase in methionine metabolites in the mutant gut that might be allowed by higher levels of nicotinamides. The steroid pathway was also enriched in tert−/− no Cre fish. Not only the stress hormone cortisol but also female hormones (such as 16-oxoestrone or estradiol) were elevated in tert−/− no Cre male fish (Extended Data Fig. 6b). Overall, our unbiased metabolomics analysis described an altered metabolism profile in tert−/− no Cre that was recovered by gut-specific telomerase expression.
Local effects
Gut tert rescues gut microbiota dysbiosis
Gut microbiota dysbiosis is associated with a dysfunctional intestinal barrier and is suggested to generate a feed-forward loop involving gut permeability, inflammation and dysbiosis in aging31,32. However, it was unclear whether delaying gut aging would counteract gut microbiota dysbiosis. To investigate if telomerase expression in the gut of tert−/− fish ameliorates gut dysbiosis, we performed high-throughput sequencing of the V3 and V4 regions of 16S ribosomal DNA of 9-month-old zebrafish gut. As described for human aging33,34, we observed diminished microbial diversity in tert−/− no Cre when compared to WT controls. Both α (within samples) and β (within groups) analyses showed lower diversity in tert−/− no Cre individuals compared to other groups (Fig. 3a,b). According to a reduced β diversity, using principal coordinates analysis (PCoA), we observed a clustering of tert−/− no Cre samples while WT and tert−/− + Cre samples were more dispersed (Fig. 3c).
The relative abundance of bacterial taxonomic units at the class level revealed an overall alteration of gut microbiota composition in tert−/− no Cre fish that was recovered by tert expression (Fig. 3d). At the class level, we observed in the tert−/− no Cre group a decreased abundance of Alphaproteobacteria and Planctomycetes along with an enrichment in Gammaproteobacteria, Bacteroidia and Fibrobacteria (Fig. 3d and Extended Data Fig. 7a). While Alphaproteobacteria inhibit host cell death and promote proliferation35, Gammaproteobacteria expansion is associated with early age-dependent loss of intestinal barrier integrity in flies32. Similarly, at the genus level, the Alphaproteobacteria Reyranella and Defluviimonas were reduced while the Gammaproteobacteria Aeromonas and Shewanella along with Bacteroides, a Bacteroidia-related genus, were enriched in tert−/− no Cre fish (Fig. 3e and Extended Data Fig. 7b). Both Shewanella and Aeromonas have been described as deleterious in humans, with Shewanella causing intra-abdominal infections36 and Aeromonas being associated with inflammatory bowel disease and inflammation37,38. Within the Aeromonas genus, Aeromonas veronii was strikingly overrepresented in tert−/− no Cre fish (Extended Data Fig. 7c). From the Bacteroidia class, Bacteroides uniformis, Parabacteroides merdae and Bacteroides ovatus were similarly enriched in tert−/− no Cre and are considered ‘pathobionts’ that profit from a dysregulated environment to overtake commensal symbionts and become pathogenic39,40,41. Overall, our analysis of gut microbiota composition revealed a wrongly balanced gut microbiome in tert−/− no Cre fish, containing a less diverse bacterial community with increased representation of otherwise pathogenic taxa microbiota being more pathogenic. These features were reverted by gut-specific telomerase expression.
Systemic effects
Gut tert rescues tissue degeneration
Intestinal dysfunction is a major feature of aging4. To investigate whether gut aging influences overall organismal aging, we explored the systemic impact of gut-specific telomerase expression using histological analyses of a broad spectrum of tissues. As reported previously14,16,17, we observed a reduction in mature spermatids area (with severe testes atrophy), in adipocyte size in visceral adipose tissues, in muscle fiber thickness and in retinal pigmented epithelium width in the tert−/− no Cre fish compared to WT (Fig. 4a,b). Strikingly, gut-specific telomerase expression recovered all these morphological defects. Of note, no histological differences were detected in kidney marrow between each condition. Moreover, unlike previous observations14,16, the adipocyte size of subcutaneous adipose tissue and the photoreceptor layer did not differ between the three genotypes. Therefore, our results indicate that counteracting telomere shortening in the gut systemically ameliorates age-dependant tissue degeneration.
Gut short telomeres drive systemic DNA damage and inflammation
We decided to study the extent of systemic aging recovery of specific distant organs. Given the importance of anemia in patients with TBD42,43, and the drastic histological phenotype seen in the testes of tert−/− no Cre fish, we further detailed the rescue in the kidney marrow (the adult hematopoietic organ in zebrafish) and testes (the reproductive system). As described previously14,17, we observed increased γH2AX-positive cells and high levels of p53 in both the testes and kidney marrow of tert−/− no Cre fish (Figs. 5a,b and 6a,b). These organs were affected by reduced cell proliferation and high senescence (Figs. 5c–f and 6c–f). In the kidney, even though most cells affected by DNA damage and low proliferation reside in the hematopoietic compartment, we also observed SA-β-Gal-positive cells in kidney tubules, suggesting that both hematopoietic and nephrotic functions were affected in tert−/− no Cre fish.
Surprisingly, gut-specific telomerase expression in tert−/− mutants resulted in a reduction in DNA damage, p53 levels and recovery of cell proliferation in both testes and kidney marrow (Figs. 5a–c and 6a–c). Moreover, SA-β-Gal and ink4a/b (p15/16) mRNA levels were reduced to WT levels in tert−/− + Cre testes and kidney marrow (Figs. 5d,e and 6d,e). While cdkn1a (p21) mRNA levels were maintained in the testes of tert−/− no Cre fish, these were rescued in kidney marrow of tert−/− + Cre fish (Figs. 5f and 6f). Consistent with what we observed in the gut, apoptosis did not vary in either testes or kidney marrow (Extended Data Fig. 2b,c). Therefore, gut-specific telomerase expression unexpectedly rescues DNA damage, proliferation and senescence in both the reproductive and hematopoietic systems.
The increased immune infiltrates present in the testes of tert−/− no Cre fish were also reverted in the tert−/− + Cre fish (Fig. 5g,h). However, in contrast to the gut and testes, we observed a considerable reduction of immune cells in the kidney marrow of tert−/− no Cre fish (Fig. 6g,h). These numbers were reverted to WT levels in tert−/− + Cre fish. Thus, our results provide evidence for a decreased reserve pool of immune cells in tert−/− no Cre fish that is rescued by gut-specific telomerase expression. Decline of immune cells in the kidney marrow constitutes an early sign of hematopoietic dysfunction, which is comparable to the bone marrow failure described in patients with TBD42,43.
To ensure that these effects were not due to leaky fabp2 enterocyte promoter expression in other tissues, we performed quantitative PCR with reverse transcription (RT–qPCR) experiments on testes and kidney marrow. While a clear induction of the tert transgene and total tert mRNA was observed in the gut of tert−/− + Cre fish, no expression of the transgene was detected in either distant organ (Extended Data Fig. 1a,b). Consistently, the DsRed reporter for transgene expression showed that the fabp2 promoter was solely expressed in gut differentiated cells but not the testes or kidney marrow (Extended Data Fig. 4). As expected, we observed neither telomerase activity nor telomere elongation in distant organs (Extended Data Fig. 1g–p). In contrast, telomere shortening was observed in tert−/− + Cre kidney marrow and testes, similar to the telomere length of tert−/− no Cre fish. These experiments support a systemic role of gut-specific telomerase expression.
Fertility decreases during natural aging of zebrafish and most mammals. Loss of male fertility is accelerated in murine and fish premature tert−/− aging models14,44. To test the male reproductive function, we crossed 9-month-old males of the three groups with young WT females. The percentages of fertilized eggs spawned by young females were scored as a male fertility index. Consistent with a reduction of mature spermatid content, tert−/− no Cre male fish exhibited a drastic reduction of fertility (Fig. 5j). In contrast, we observed a full recovery of male fertility in tert−/− + Cre fish. Therefore, gut-specific telomerase expression not only improves cellular and morphological defects of the male reproductive system, but also rescues age-dependent loss of fertility.
Finally, to understand the mechanism through which gut decline influences aging of distant organs, we analyzed the transcriptomics profile of testes and kidney marrow. As in the gut, we observed similar GSEA hallmark profiles when comparing tert−/− no Cre to either WT or tert−/− + Cre (Figs. 5i and 6i), indicating that telomerase expression in the gut rescues the transcriptomics profile of tert mutant testes and kidney marrow. As in the gut, we observed a marked enrichment of hallmarks of inflammation and a reduction of proliferation-related genes in tert−/− no Cre. Hallmarks of metabolic pathways were also upregulated in tert−/− no Cre indicating a metabolic shift in this condition. Unexpectedly, even though senescence was higher in all organs of tert−/− no Cre fish (Figs. 5d–f and 6d–f), in contrast to the gut, we observed a downregulation of the SASP hallmark in both the testes and kidney marrow. This result suggests that paracrine senescence of distant organs initiated by the gut may have limited expression of SASP molecules, as previously observed in secondary senescent cells45.
Gut tert extends tert
−/− lifespan and improves WT healthspan
We next tested whether telomerase expression in the gut would influence the lifespan of zebrafish. As described previously14,15,16, telomere shortening in tert−/− no Cre fish reduces the average lifespan to 12–18 months compared to more than 42 months in WT fish (Fig. 7). Strikingly, delaying gut aging was sufficient to extend the average lifespan of tert−/− fish by approximately 40%. The average lifespan of tert−/− no Cre fish was extended from 17 months to 24 months in tert−/− + Cre fish (Fig. 7). Nevertheless, telomerase expression in the gut was not sufficient to fully rescue life expectancy to WT levels, suggesting that telomere shortening in other organs may be limiting in later stages.
Finally, to extend our discovery to the natural aging of zebrafish, we studied the recovery of 24–27-month-old WT zebrafish expressing (WT + Cre) or not expressing (WT no Cre) the tert transgene in the gut. At that age, we did not yet distinguish differences in survival between the two groups (Fig. 8d). However, we observed that gut-specific telomerase expression in WT fish increased cell proliferation, reduced gut lamina propria width and counteracted cell senescence in the gut compared to WT no Cre (Fig. 8a–c). As observed in tert−/− fish, expressing telomerase in the gut of WT fish is sufficient to improve the proliferative capacity of distant organs such as the testes and kidney marrow (Fig. 8a). Except for a partial rescue of ink4a/b (p15/16) mRNA levels in kidney marrow, we did not observed signs of senescence in either distant organ using SA-β-Gal assays or assessing for transcription levels of ink4a/b (p15/16) and cdkn1a (p21) (Fig. 8b). Consistently, we did not observe histological defects in distant organs (Fig. 8c). Therefore, while 24–27-month-old WT fish do not fully exhibit natural aging phenotypes, our data revealed that delaying gut aging by gut-specific tert overexpression is sufficient to counteract the early signs of aging, such as loss of proliferative capacity. It also confirms that the gut is one of the earliest organs affected in natural aging.