Health
Hong Kong: BioNTech, distributer find no vaccine safety issues in initial probe – Mint
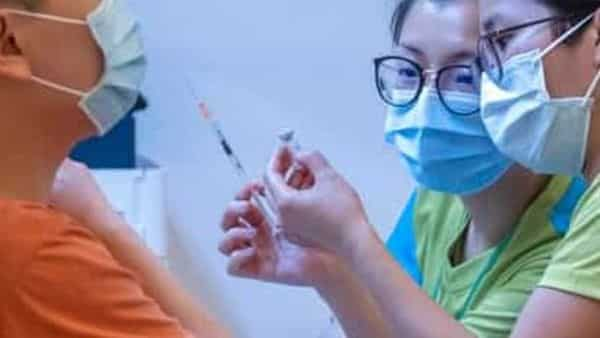
Initial investigations by BioNTech SE and its regional distributor found no safety concerns with batches of vaccines sent to Hong Kong, after packaging defects prompted a halt in the use of the Covid-19 vaccine in the city.
Early findings didn’t rule out the possibility that packaging defects may have been because of environmental factors during long-haul transport, the Hong Kong government said in a statement, without specifying. The government, BioNTech and Shanghai Fosun Pharmaceutical Group Co. aim to conclude the investigation within a week, it said.
Hong Kong Suspends BioNTech on Loose Vial Caps, Stains
The city’s vaccination campaign suffered a setback this week when it and neighboring Macau temporarily suspended shots manufactured by BioNTech because of packaging defects. The suspension risks eroding public confidence in the inoculation, which had provided Hong Kong residents an alternative to the one made by Chinese firm Sinovac Biotech Ltd.
The defects, which included loose vial caps and stained bottles, affected about 1.3 million doses that were delivered to the financial hub. About 150,000 people had received BioNTech shots in Hong Kong prior to the halt.
Fosun and BioNTech have studied the entire supply chain, including the sealing process in BioNTech’s German facilities, vaccine packaging, transport to Hong Kong, logistical processing and storage after their arrival in the city, as well as inspections at community vaccination centers. They found no “obvious systematic factors” that could have led to the defects from packaging to usage, according to the statement.
The companies also said that they didn’t believe the defects were results of cold chain and logistic management issues. Random testing of remaining vaccine vials with intact packaging didn’t find evidence of leakage.
For more articles like this, please visit us at bloomberg.com
©2021 Bloomberg L.P.
Health
RCMP warn about benzodiazepine-laced fentanyl tied to overdose in Alberta – Edmonton Journal


Article content
Grande Prairie RCMP issued a warning Friday after it was revealed fentanyl linked to a deadly overdose was mixed with a chemical that doesn’t respond to naloxone treatment.
The drugs were initially seized on Feb. 28 after a fatal overdose, and this week, Health Canada reported back to Mounties that the fentanyl had been mixed with Bromazolam, which is a benzodiazepine.
Article content
Mounties say this is the first recorded instance of Bromazolam in Alberta. The drug has previously been linked to nine fatal overdoses in New Brunswick in 2022.
The pills seized in Alberta were oval-shaped and stamped with “20” and “SS,” though Mounties say it can come in other forms.
Naloxone treatment, given in many cases of opioid toxicity, is not effective in reversing the effects of Bromazalam, Mounties said, and therefore, any fentanyl mixed with the benzodiazepine “would see a reduced effectiveness of naloxone, requiring the use of additional doses and may still result in a fatality.”
From January to November of last year, there were 1,706 opioid-related deaths in Alberta, and 57 linked to benzodiazepine, up from 1,375 and 43, respectively, in 2022.
Mounties say officers responded to about 1,100 opioid-related calls for service, last year with a third of those proving fatal. RCMP officers also used naloxone 67 times while in the field, a jump of nearly a third over the previous year.
Recommended from Editorial
Share this article in your social network
Health
CFIA continues surveillance for HPAI in cattle, while sticking with original name for disease – RealAgriculture


The Canada Food Inspection Agency will continue to refer to highly pathogenic avian influenza in cattle as HPAI in cattle, and not refer to it as bovine influenza A virus (BIAV), as suggested by the American Association of Bovine Practitioners earlier this month.
Dr. Martin Appelt, senior director for the Canadian Food Inspection Agency, in the interview below, says at this time Canada will stick with “HPAI in cattle” when referencing the disease that’s been confirmed in dairy cattle in multiple states in the U.S.
The CFIA’s naming policy is consistent with the agency’s U.S. counterparts’, as the U.S. Animal and Plant Health Inspection Service has also said it will continue referring to it as HPAI or H5N1.
Appelt explains how the CFIA is learning from the U.S. experience to-date, and how it is working with veterinarians across Canada to stay vigilant for signs of the disease in dairy and beef cattle.
As of April 19, there has not been a confirmed case of HPAI in cattle in Canada. Appelt says it’s too soon to say if an eventual positive case will significantly restrict animal movement, as is the case with positive poultry cases.
This is a major concern for the cattle industry, as beef cattle especially move north and south across the U.S. border by the thousands. Appelt says that CFIA will address an infection in each species differently in conjunction with how the disease is spread and the threat to neighbouring farms or livestock.
Currently, provincial dairy organizations have advised producers to postpone any non-essential tours of dairy barns, as a precaution, in addition to other biosecurity measures to reduce the risk of cattle contracting HPAI.
Subscribe: Apple Podcasts | Spotify | RSS | All Podcasts
jQuery(document).ready(function($) {
$(“#homesub”).validate(
rules:
first_name:
required: true,
minlength: 2
,
last_name:
required: true,
minlength: 2
,
email:
required: true,
email: true,
minlength: 2
,
state:
required: true,
,
role:
required: true,
,
“listid[]”:
required: true,
minlength: 1
,
messages:
first_name: “Your first name is required.”,
last_name: “Your last name is required.”,
email: “Please verify your email is correct.”,
state: “Your state/province is required.”,
role: “Your role is required.”,
“listid[]”: “Select at least one list is required.”
,
submitHandler: function()
$.ajax(
type: “POST”,
url: “https://www.realagriculture.com/wp-admin/admin-ajax.php”,
data:
action: “realag_cc_process_subscribe_onclick”,
form: “homesub”,
data: $(“#homesub”).serialize(),
,
dataType: “html”,
timeout: 30000,
error: function(response)
console.log(response);
,
success: function(response)
$(“#homesub”).html(response);
,
);
);
});
Health
Toronto reports 2 more measles cases. Use our tool to check the spread in Canada – Toronto Star

/* OOVVUU Targeting */
const path = ‘/news/canada’;
const siteName = ‘thestar.com’;
let domain = ‘thestar.com’;
if (siteName === ‘thestar.com’)
domain = ‘thestar.com’;
else if (siteName === ‘niagarafallsreview.ca’)
domain = ‘niagara_falls_review’;
else if (siteName === ‘stcatharinesstandard.ca’)
domain = ‘st_catharines_standard’;
else if (siteName === ‘thepeterboroughexaminer.com’)
domain = ‘the_peterborough_examiner’;
else if (siteName === ‘therecord.com’)
domain = ‘the_record’;
else if (siteName === ‘thespec.com’)
domain = ‘the_spec’;
else if (siteName === ‘wellandtribune.ca’)
domain = ‘welland_tribune’;
else if (siteName === ‘bramptonguardian.com’)
domain = ‘brampton_guardian’;
else if (siteName === ‘caledonenterprise.com’)
domain = ‘caledon_enterprise’;
else if (siteName === ‘cambridgetimes.ca’)
domain = ‘cambridge_times’;
else if (siteName === ‘durhamregion.com’)
domain = ‘durham_region’;
else if (siteName === ‘guelphmercury.com’)
domain = ‘guelph_mercury’;
else if (siteName === ‘insidehalton.com’)
domain = ‘inside_halton’;
else if (siteName === ‘insideottawavalley.com’)
domain = ‘inside_ottawa_valley’;
else if (siteName === ‘mississauga.com’)
domain = ‘mississauga’;
else if (siteName === ‘muskokaregion.com’)
domain = ‘muskoka_region’;
else if (siteName === ‘newhamburgindependent.ca’)
domain = ‘new_hamburg_independent’;
else if (siteName === ‘niagarathisweek.com’)
domain = ‘niagara_this_week’;
else if (siteName === ‘northbaynipissing.com’)
domain = ‘north_bay_nipissing’;
else if (siteName === ‘northumberlandnews.com’)
domain = ‘northumberland_news’;
else if (siteName === ‘orangeville.com’)
domain = ‘orangeville’;
else if (siteName === ‘ourwindsor.ca’)
domain = ‘our_windsor’;
else if (siteName === ‘parrysound.com’)
domain = ‘parrysound’;
else if (siteName === ‘simcoe.com’)
domain = ‘simcoe’;
else if (siteName === ‘theifp.ca’)
domain = ‘the_ifp’;
else if (siteName === ‘waterloochronicle.ca’)
domain = ‘waterloo_chronicle’;
else if (siteName === ‘yorkregion.com’)
domain = ‘york_region’;
let sectionTag = ”;
try
if (domain === ‘thestar.com’ && path.indexOf(‘wires/’) = 0)
sectionTag = ‘/business’;
else if (path.indexOf(‘/autos’) >= 0)
sectionTag = ‘/autos’;
else if (path.indexOf(‘/entertainment’) >= 0)
sectionTag = ‘/entertainment’;
else if (path.indexOf(‘/life’) >= 0)
sectionTag = ‘/life’;
else if (path.indexOf(‘/news’) >= 0)
sectionTag = ‘/news’;
else if (path.indexOf(‘/politics’) >= 0)
sectionTag = ‘/politics’;
else if (path.indexOf(‘/sports’) >= 0)
sectionTag = ‘/sports’;
else if (path.indexOf(‘/opinion’) >= 0)
sectionTag = ‘/opinion’;
} catch (ex)
const descriptionUrl = ‘window.location.href’;
const vid = ‘mediainfo.reference_id’;
const cmsId = ‘2665777’;
let url = `https://pubads.g.doubleclick.net/gampad/ads?iu=/58580620/$domain/video/oovvuu$sectionTag&description_url=$descriptionUrl&vid=$vid&cmsid=$cmsId&tfcd=0&npa=0&sz=640×480&ad_rule=0&gdfp_req=1&output=vast&unviewed_position_start=1&env=vp&impl=s&correlator=`;
url = url.split(‘ ‘).join(”);
window.oovvuuReplacementAdServerURL = url;
Canada has seen a concerning rise in measles cases in the first months of 2024.
By the third week of March, the country had already recorded more than three times the number of cases as all of last year. Canada had just 12 cases of measles in 2023, up from three in 2022.
function buildUserSwitchAccountsForm()
var form = document.getElementById(‘user-local-logout-form-switch-accounts’);
if (form) return;
// build form with javascript since having a form element here breaks the payment modal.
var switchForm = document.createElement(‘form’);
switchForm.setAttribute(‘id’,’user-local-logout-form-switch-accounts’);
switchForm.setAttribute(‘method’,’post’);
switchForm.setAttribute(‘action’,’https://www.thestar.com/tncms/auth/logout/?return=https://www.thestar.com/users/login/?referer_url=https%3A%2F%2Fwww.thestar.com%2Fnews%2Fcanada%2Ftoronto-reports-2-more-measles-cases-use-our-tool-to-check-the-spread-in-canada%2Farticle_20aa7df4-e88f-11ee-8fad-8f8368d7ff53.html’);
switchForm.setAttribute(‘style’,’display:none;’);
var refUrl = document.createElement(‘input’); //input element, text
refUrl.setAttribute(‘type’,’hidden’);
refUrl.setAttribute(‘name’,’referer_url’);
refUrl.setAttribute(‘value’,’https://www.thestar.com/news/canada/toronto-reports-2-more-measles-cases-use-our-tool-to-check-the-spread-in-canada/article_20aa7df4-e88f-11ee-8fad-8f8368d7ff53.html’);
var submit = document.createElement(‘input’);
submit.setAttribute(‘type’,’submit’);
submit.setAttribute(‘name’,’logout’);
submit.setAttribute(‘value’,’Logout’);
switchForm.appendChild(refUrl);
switchForm.appendChild(submit);
document.getElementsByTagName(‘body’)[0].appendChild(switchForm);
function handleUserSwitchAccounts()
window.sessionStorage.removeItem(‘bd-viafoura-oidc’); // clear viafoura JWT token
// logout user before sending them to login page via return url
document.getElementById(‘user-local-logout-form-switch-accounts’).submit();
return false;
buildUserSwitchAccountsForm();
#ont-map-iframepadding:0;width:100%;border:0;overflow:hidden;
#ontario-cases-iframepadding:0;width:100%;border:0;overflow:hidden;
#province-table-iframepadding:0;width:100%;border:0;overflow:hidden;
console.log(‘=====> bRemoveLastParagraph: ‘,0);
-
Media17 hours ago
DJT Stock Rises. Trump Media CEO Alleges Potential Market Manipulation. – Barron's
-
Media19 hours ago
Trump Media alerts Nasdaq to potential market manipulation from 'naked' short selling of DJT stock – CNBC
-
Investment17 hours ago
Private equity gears up for potential National Football League investments – Financial Times
-



 Sports22 hours ago
Sports22 hours ago2024 Stanley Cup Playoffs 1st-round schedule – NHL.com
-
News16 hours ago
Canada Child Benefit payment on Friday | CTV News – CTV News Toronto
-
Real eState9 hours ago
Botched home sale costs Winnipeg man his right to sell real estate in Manitoba – CBC.ca
-
Business18 hours ago
Gas prices see 'largest single-day jump since early 2022': En-Pro International – Yahoo Canada Finance
-
Art21 hours ago
Enter the uncanny valley: New exhibition mixes AI and art photography – Euronews







