Health
If I have allergies, should I get the coronavirus vaccine? An expert answers this and other questions – The Conversation US
Editor’s Note: With a coronavirus vaccination effort now underway, you might have questions about what this means for you and your family. If you do, send them to The Conversation, and we will find a physician or researcher to answer them. Here, Dr. Mona Hanna-Attisha, a public health pediatrician whose research exposed the Flint, Michigan, water crisis, answers questions about the vaccine and allergies, and when kids might be able to get the vaccine.
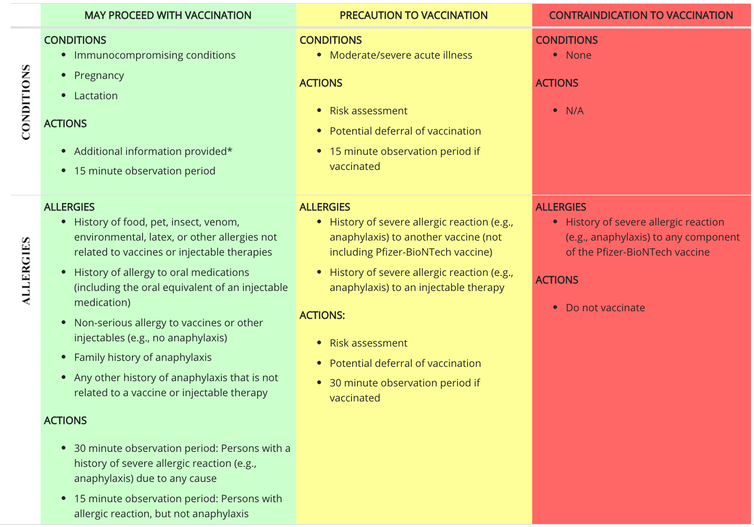
If I have allergies, should I still get the vaccine?
If you have a history of allergies to food, pets, insects or other things, the Centers for Disease Control and Prevention recommends that you proceed with vaccination, with an observation period. If you have a history of severe allergic reaction, or what is called anaphylaxis, to another vaccine or injectable therapy, your doctor can do a risk assessment, defer your vaccination, or proceed and then observe you after vaccination. The only reason to avoid vaccination is a severe allergic reaction to any component of the COVID-19 vaccine. The CDC has specific recommendations for post-vaccine observation.
As the vaccine goes out to a broader population, how will adverse events be tracked?
The CDC and Food and Drug Administration encourage the public to report possible adverse events to the Vaccine Adverse Event Reporting System, or VAERS. This national system collects these data to look for adverse events that are unexpected, appear to happen more often than expected or have unusual patterns of occurrence. Anyone who has experienced an adverse event should report it to the system.
Reporting an adverse event is a crucial step to ensuring safety and to help the CDC monitor the vaccines. Safety is a top priority, and scientists and public health officials need to know about adverse reactions.
An adverse event is different in most cases from a typical vaccine side effect. Vaccines may cause a side effect, such as soreness at the injection site or redness. Adverse events are more serious and can sometimes be life-threatening. If you are unsure whether you have experienced a side effect or adverse event, you can still report the event.
Participants are given a fact sheet when they are vaccinated. Health care providers who vaccinate people will be required to report to VAERS certain adverse events following vaccination. In addition, under the terms of the emergency use authorization, health care providers also must follow any revised safety reporting requirements that may arise.
The CDC is also implementing a new smartphone-based tool called v-safe to check in on people’s health after they receive a COVID-19 vaccine. When you receive your vaccine, you should also receive an information sheet telling you how to enroll in v-safe. If you enroll, you will receive regular text messages directing you to surveys where you can report any problems or adverse reactions you have after receiving a COVID-19 vaccine.
CDC
When might kids younger than 16 be vaccinated?
It is likely to be several months. The currently authorized Pfizer and soon-to-be-authorized Moderna vaccine are not applicable for children. More research and clinical trials need to be done to include younger children in COVID-19 vaccine trials.
According to the American Academy of Pediatrics, Pfizer has enrolled children down to age 12 and submitted a request for emergency use authorization for vaccination down to age 16. Moderna, whose vaccine is expected to receive emergency use authorization from the FDA any day, is about to start a similar study.
In the United Kingdom, AstraZeneca has approval to enroll children ages 5 to 12 in clinical trials, but the pharmaceutical company has not yet enrolled any children in trials in the U.S.
Health
Whooping cough cases up slightly in N.L., as officials warn about risks to infants – CBC.ca

Newfoundland and Labrador’s top doctor is warning people to stay up to date on whooping cough vaccinations after a small increase in cases this year.
The province usually sees three to four cases of the disease annually. Up to 10 cases have been reported already since January, however, prompting the province’s chief medical officer to raise the issue publicly.
The increase “generally means there’s a little bit more circulating in the community than what’s presenting for care and testing,” Dr. Janice Fitzgerald said Tuesday.
While officials aren’t overly concerned about a future spike in cases, Fitzgerald said, higher infection rates place infants in particular at risk.
Children under the age of one aren’t yet old enough for the whooping cough vaccine and don’t have immunity to the disease, Fitzgerald said. Infections in small children can be more severe and lead to pneumonia, neurological issues and hospitalization.
Fitzgerald said parents, grandparents and caregivers should check to ensure their vaccinations are up to date.
Whooping cough, also known as pertussis, causes a persistent nagging cough that’s sometimes severe enough to cause vomiting. Vaccines for the disease are offered in early childhood, during high school and in adulthood. Booster shots should be given 10 years after the high school dose, Fitzgerald said.
“Immunity can wane over time,” she said. “Pertussis does circulate on a regular basis in our community.”
The small increase in cases isn’t yet ringing alarm bells for undervaccination within the general population, she added, noting the province still has a vaccination rate over 90 per cent.
Download our free CBC News app to sign up for push alerts for CBC Newfoundland and Labrador. Click here to visit our landing page.
Health
Supervised consumption sites urgently needed, says study – Sudbury.com

A study in the Canadian Medical Association Journal (CMAJ) said the opioid drug crisis has reached such a critical level that a public safety response is urgently required and that includes the need for expanded supervised consumption sites.
The report was published by the medical journal Monday and was authored by Shaleesa Ledlie, David N. Juurlink, Mina Tadrous, Muhammad Mamdani, J. Michael Paterson and Tara Gomes; physicians and scientists associated with the University of Toronto, Sunnybrook Research Institute and the Li Ka Shing Knowledge Institute at St. Michael’s Hospital.
“The drug toxicity crisis continues to accelerate across Canada, with rapid increases in opioid-related harms following the onset of the COVID-19 pandemic,” the authors wrote. “We sought to describe trends in the burden of opioid-related deaths across Canada throughout the pandemic, comparing these trends by province or territory, age and sex.”
The study determined that across Canada, the burden of premature opioid-related deaths doubled between 2019 and 2021, representing more than one-quarter of deaths among younger adults. The disproportionate loss of life in this demographic group highlights the critical need for targeted prevention efforts, said the study.
The researchers found that the death rate increased significantly as fentanyl was introduced to the mix of street drugs that individuals were using, in some cases, unknowingly.
The authors said this demonstrates the need for consumption sites, not only as overwatch as people with addictions consume their drugs, but also to make an effort to identify the substances and inform those people beforehand.
“The increased detection of fentanyl in opioid-related deaths in Canada highlights the need for expansion of harm-reduction programs, including improved access to drug-checking services, supervised consumption sites, and treatment for substance use disorders,” the authors wrote.
The study said a more intense public safety response is needed.
“Given the rapidly evolving nature of the drug toxicity crisis, a public safety response is urgently required and may include continued funding of safer opioid supply programs that were expanded beginning in March 2020, improved flexibility in take-home doses of opioid agonist treatment, and enhanced training for health care workers, harm reduction workers, and people who use drugs on appropriate responses to opioid toxicities involving polysubstance use.
In conclusion, the authors wrote that during the height of the COVID pandemic in 2020 and 2021, the burden of premature death from accidental opioid toxicities in Canada dramatically increased, especially in Alberta, Saskatchewan, and Manitoba.
“In 2021, more than 70 per cent of opioid-related deaths occurred among males and about 30 per cent occurred among people aged 30–39 years, representing one in every four deaths in this age group. The disproportionate rates of opioid-related deaths observed in these demographic groups highlight the critical need for the expansion of targeted harm reduction–based policies and programs across Canada,” said the study.
The full text of the report can be found online here.
Health
Business Plan Approved for Cancer Centre at NRGH – My Cowichan Valley Now


A business plan for a new BC Cancer Centre at Nanaimo Regional General Hospital has been approved by the province.
Health Minister Adrian Dix says the state-of-the-art cancer facility will benefit patients in Nanaimo and the surrounding region through the latest medical technology.
The facility will have 12 exam rooms, four consultation rooms and space for medical physicists and radiation therapists, medical imaging and radiation treatment of cancer patients.
The procurement process is underway, and construction is expected to begin in 2025 and be complete in 2028.
Upgrades to NRGH have also been approved, such as a new single-storey addition to the ambulatory care building and expanded pharmacy.
Dix says Nanaimo’s population is growing rapidly and aging, and stronger health services in the region, so people get the health care they need closer to home.
-
News22 hours ago
Loblaws Canada groceries: Shoppers slam store for green onions with roots chopped off — 'I wouldn't buy those' – Yahoo News Canada
-
Business21 hours ago
Rupture on TC Energy's NGTL gas pipeline sparks wildfire in Alberta – The Globe and Mail
-
Investment21 hours ago
Saudi Arabia Highlights Investment Initiatives in Tourism at International Hospitality Investment Forum
-



 Tech14 hours ago
Tech14 hours agoCytiva Showcases Single-Use Mixing System at INTERPHEX 2024 – BioPharm International
-
Art21 hours ago
Squatters at Gordon Ramsay's Pub Have 'Left the Building' After Turning It Into an Art Café – PEOPLE
-



 Politics21 hours ago
Politics21 hours agoThe Earthquake Shaking BC Politics
-


 Sports24 hours ago
Sports24 hours agoGame in 10: Maple Leafs squander multi-goal lead to Florida, draw the Boston Bruins in the first round – Maple Leafs Hot Stove
-



 Investment20 hours ago
Investment20 hours agoBill Morneau slams Freeland’s budget as a threat to investment, economic growth




