Health
Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials
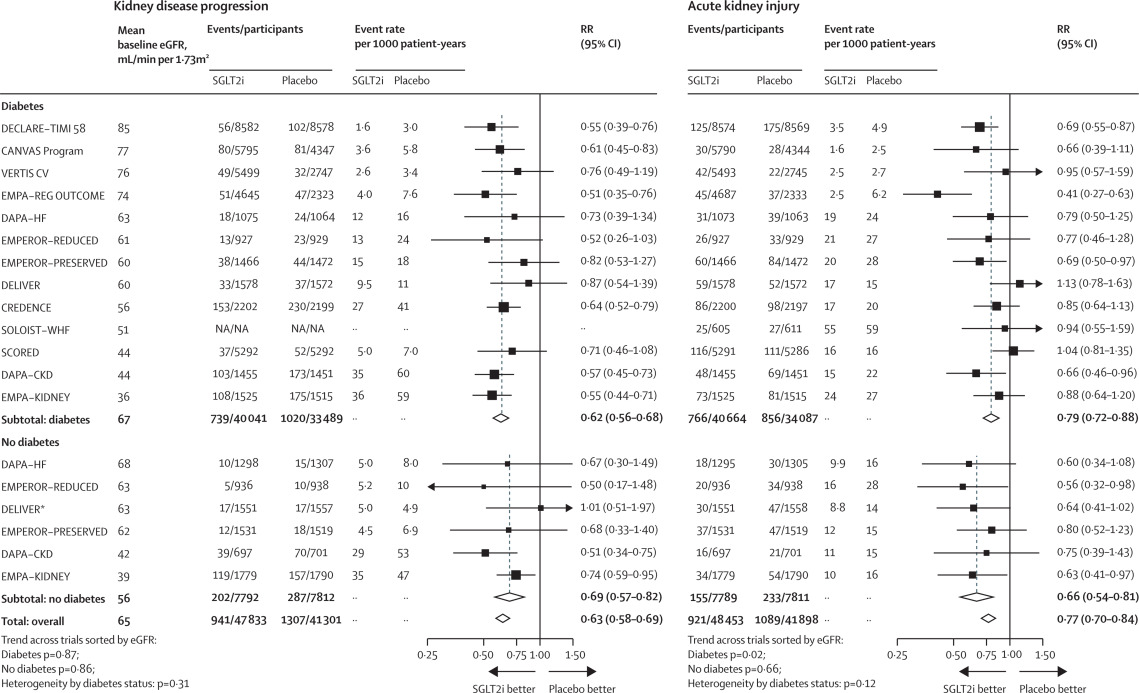
|
|
Declaration of interests
NS, RH, KJM, AJR, SYAN, DZ, PJ, DP, MJL, CB, JRE, and WGH report institutional grant funding from Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial. NS additionally reports institutional grant funding from Novo Nordisk. RH additionally reports institutional grant funding from Novartis; and trial drug supply from Roche and Regeneron. CB additionally reports grant funding from the UK Medical Research Council, the UK National Institute for Health and Care Research Health Technology Assessment, and Health Data Research UK; and advisory roles for Merck, the National Institute for Health and Care Research Health Technology Assessment, the British Heart Foundation, and the European Society of Cardiology. WGH additionally reports funding from the UK Medical Research Council–Kidney Research UK Professor David Kerr Clinician Scientist Award. BLN reports consultancy fees and honorarium paid to his institution by AstraZeneca, Bayer, Boehringer Ingelheim, Cambridge Healthcare Research, American Diabetes Association, Renal Society of Australasia and Janssen; and advisory board membership (fees paid to institution) with AstraZeneca, Bayer, and Boehringer Ingelheim. SJH and MB are full-time employees of Boehringer Ingelheim International. SDA reports institutional grant funding from Vifor Int and Abbott Vascular; consultancy or advisory board fees from CVRx, Amgen, Respicardia, Novo Nordisk, Brahms, Novartis, Sanofi, and Cordio; and additional leadership or advisory board roles with Vifor Int, Bayer, Boehringer Ingelheim, Servier, Abbott Vascular, Impulse Dynamics, AstraZeneca, Bioventrix, Janssen, Cardior, V-Wave, Cardiac Dimensions, and Occlutech. JB reports consultancy fees and honorarium from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sequana Medical, and Vifor. DZIC reports institutional grant funding from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca, CSL-Behring, and Novo Nordisk; and consultancy fees and honorarium from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie, Janssen, Bayer, Prometic, Bristol Myers Squibb, Maze, Gilead, CSL-Behring, Otsuka, Novartis, Youngene, Lexicon, and Novo Nordisk. JBG reports institutional grant funding from Boehringer Ingelheim-Lilly, Merck, Roche, and Sanofi and Lexicon; and consultancy fees from Boehringer Ingelheim-Lilly, Bayer, AstraZeneca, Sanofi and Lexicon, Hawthorne Effect and Omada, Pfizer, Valo, Anji, Vertex, and Novo Nordisk. C-CL is an employee of Merck Sharp & Dohme (a subsidiary of Merck & Co) and owns stock and/or stock options in Merck & Co. FRMcC reports grant funding from NIDDK, Satellite Healthcare, Advanced Medical, and Fifth Eye; and consultancy fees from GlaxoSmithKline, Advanced Medical, and Zydus Therapeutics. DKMcG reports consultancy fees from Merck & Co, Applied Therapeutics, Metavant, Sanofi, Afimmune, Lilly USA, Boehringer Ingelheim, Novo Nordisk, Bayer, GlaxoSmithKline, Lexicon, Altimmune, and Esperion; and other honorarium from Kirkland & Ellis, Pfizer, GlaxoSmithKline, Janssen, Afimmune, Sanofi, Boehringer Ingelheim, Merck & Co, AstraZeneca, Novo Nordisk, Esperion, and Lilly USA. JJVMcM reports institutional grant funding from AstraZeneca; consultancy fees from Abbott, Alkem Metabolics, Eris Lifesciences, Hikma, Lupin, Sun Pharmaceuticals, Heart.Org (Medscape Cardiology), ProAdWise Communications, Radcliffe Cardiology, Servier, and The Corpus; and fees paid to his institution for other advisory roles by Cytokinetics, Amgen, AstraZeneca, Theracos, Ionis Pharmaceuticals, DalCor, Cardurion, Novartis, GlaxoSmithKline, Bayer, KBP Biosciences, Boehringer Ingelheim, and Bristol Myers Squibb. MP reports personal fees from AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Moderna, Novartis, Reata, Relypsa, and Salamandra. VP reports consultancy fees, honorarium, or advisory roles supported by AbbVie, Bayer, Boehringer Ingelheim, Chinook, GlaxoSmithKline, Janssen, Pfizer, AstraZeneca, Baxter, Eli Lilly, Gilead, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Retrophin, Roche, Sanofi, Servier, and Vitae. MSS reports institutional grant funding from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Daiichi-Sankyo, Eisai, Intarcia, Ionis, Medicines Company, MedImmune, Merck, Novartis, Pfizer, and Quark Pharmaceuticals; and consultancy fees from Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol Myers Squibb, and DalCor. SDS reports institutional grant funding from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, the National Heart, Lung, and Blood Institute (US National Institutes of Health), Neurotronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI; and consultancy fees from Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, and Puretech Health. MV reports grant funding or advisory board fees from Amgen, AstraZeneca, American Regent, Baxter HealthCare, Bayer, Boehringer Ingelheim, Cytokinetics, Pharmacosmos, Relypsa, Novartis, Roche Diagnostics, Lexicon Pharmaceuticals, Galmed, Occlutech, Impulse Dynamics, Sanofi, and Tricog Health; speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics; and actively participates on clinical trial committees for studies sponsored by Galmed, Novartis, Bayer, Occlutech, and Impulse Dynamics. CW reports institutional grant funding from Boehringer Ingelheim; and consultancy fees and honorarium from Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, and Bayer. SDW reports institutional grant funding from Abbott, Amgen, Anthos Therapeutics, ARCA Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi-Sankyo, Eisai, Intarcia, Ionis Pharmaceuticals, Janssen Research and Development, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, Softcell Medical, The Medicines Company, and Zora Biosciences; and consultancy fees from AstraZeneca, Boston Clinical Research Institute, Icon Clinical, and Novo Nordisk. FZ reports consultancy fees from Amgen, Applied therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cardior, Cereno Scientific, CEVA, Cellprothera, CVRx, Novartis, Novo Nordisk, Servier, Merck, Bristol Myers Squibb; and honorarium or other personal fees from Boehringer Ingelheim, Merck, Bayer, Vifor, Fresenius, Roche Diagnostics, Hogan and Lovells, and Acceleron. HJLH reports grant funding from AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk; consultancy fees from AstraZeneca, AbbVie, Bayer, Boehringer Ingelheim, CSL Behring, Chinook, Dimerix, Eli Lilly, Gilead, Goldfinch Bio, Merck, Novartis, Novo Nordisk, Janssen, and Travere Therapeutics; and other payment or honorarium from AstraZeneca, Novo Nordisk, and Eli Lilly. MJL additionally reports institutional grant funding from Novartis and Janssen; and trial drug supply from Roche and Regeneron.
Health
New technology to advance women's cancer care at Southlake – BarrieToday


NEWS RELEASE
SOUTHLAKE REGIONAL HEALTH CENTRE
**************************
This Cancer Awareness Month, Southlake is adding advanced technologies to detect and treat breast cancer and other women’s cancers thanks to generous community donor support, most recently through the HERE is Where Cancer Meets its Match campaign. New cancer care technology, including new mammography machines, the MyoSure System and the MOLLI 2® System will make a measurable impact in diagnosing and treating women’s cancers in the communities Southlake serves.
Southlake is installing three new mammography machines to expand its breast cancer screening program to 1,500 more women each year. Two of these machines have new biopsy capabilities that will reduce the number of cancelled exams due to equipment failure, ensuring timely care for women. Women ages 40 to 49 years old will be able to self-refer for publicly funded mammograms through the Ontario Breast Screening Program starting this fall.
“Early detection is critical when treating breast cancer and other women’s cancers,” said Lorrie Reynolds, Director, Regional Cancer Program at Southlake. “We treat more than 1,700 breast cancer patients at Southlake every year. By adding advanced technology, like the new mammography machines, we’re ensuring women have the best experience at Southlake.”
Southlake is also introducing the MyoSure System, an innovative technology that can help detect female reproductive cancers. Damaged tissue in a woman’s uterus such as fibroids and polyps can now be removed in a precise, minimally invasive procedure that leaves the rest of the uterus intact. This will improve the overall patient experience by supporting faster recovery, reducing the risk of infection and giving more women the option to have children. An estimated 200 women per year will benefit from the MyoSure System.
The new mammography machines and the MyoSure System build on Southlake’s recent investment in the MOLLI 2® System, a made-in-Canada wire-free breast localization technology. This technology is considerably less invasive and more accurate when compared to wire-guided localization, resulting in a better patient experience and improved cosmetic outcomes. More than 200 women each year will benefit from this innovative medical device as they are treated for breast cancer at Southlake.
“As a clinician caring for women with cancer in our community, I’m incredibly proud of the work Southlake is doing to advance women’s health and improve patient experiences,” said Sara Temple, MD, Surgical Oncologist and Chief of Surgery at Southlake. “Women who visit Southlake can be confident that they are receiving leading edge care, close to home when they need it most.”
The World Health Organization anticipates a 77 per cent increase in cancer diagnoses by 2050. Southlake serves some of the fastest growing communities in Canada and anticipates that the number of patients requiring cancer care will grow. By investing in new technology, Southlake is ensuring that women in the communities it serves have access to leading edge cancer care. All of these investments were funded with support from community donors who generously gave to Southlake to support investments into women’s health at the hospital.
“The generosity of our donor community and the impact they have made for women receiving cancer diagnosis and treatment at Southlake is something we can all take great pride in,” said Jennifer Ritter, President and CEO of Southlake Foundation. “From our Women’s Health Initiative donors supporting new mammography machines, to the Ladies in Philanthropy for Southlake funding the MOLLI 2 System, to our long-standing partners The Edge Benefits and Pheasant Run Golf Club enabling the introduction of MyoSure System through their joint annual charity golf tournament, we are incredibly lucky to share a vision of access to exceptional care for everyone who depends on Southlake when they need us most. Thank you, to every donor who contributed to these important upgrades to care for women.”
Southlake Foundation’s HERE is Where Cancer Meets its Match campaign supports the Stronach Regional Cancer Centre at Southlake. For more information or to make a donation, visit: southlake.ca/HERE.
**************************
Health
Pasteurized milk includes remnants of H5N1 bird flu, U.S. officials say


|
|
The U.S. Food and Drug Administration says that samples of pasteurized milk have tested positive for remnants of the bird flu virus that has infected dairy cows.
The agency stressed that the material is inactivated and that the findings “do not represent actual virus that may be a risk to consumers.” Officials added that they’re continuing to study the issue.
“To date, we have seen nothing that would change our assessment that the commercial milk supply is safe,” the FDA said in a statement on Tuesday.
The announcement comes nearly a month after an avian influenza virus that has sickened millions of wild and commercial birds in recent years was detected in dairy cows in at least eight states. The Agriculture Department (USDA) says 33 herds have been affected to date.
FDA officials didn’t indicate how many samples they tested or where they were obtained. The agency has been evaluating milk during processing and from grocery stores, officials said. Results of additional tests are expected in “the next few days to weeks.”
WATCH | Bird flu spread in U.S. cows:
For the first time ever, avian influenza, or H5N1 bird flu, was detected in roughly a dozen dairy cow herds across the U.S. About That producer Lauren Bird explores why scientists and public health officials are concerned about the cross-species transmission and whether humans are now at higher risk.
The polymerase chain reaction (PCR) lab test the FDA used would have detected viral genetic material even after live virus was killed by pasteurization, or heat treatment, said Lee-Ann Jaykus, an emeritus food microbiologist and virologist at North Carolina State University
“There is no evidence to date that this is infectious virus, and the FDA is following up on that,” Jaykus said.
Officials with the FDA and the USDA had previously said milk from affected cattle did not enter the commercial supply. Milk from sick animals is supposed to be diverted and destroyed. Federal regulations require milk that enters interstate commerce to be pasteurized.
Tests for viable virus underway, agency says
Because the detection of the bird flu virus known as Type A H5N1 in dairy cattle is new and the situation is evolving, no studies on the effects of pasteurization on the virus have been completed, FDA officials said. But past research shows that pasteurization is “very likely” to inactivate heat-sensitive viruses like H5N1, the agency added.
The agency said it has been evaluating milk from affected animals, in the processing system and on the shelves. It said it is completing a large, representative national sample to understand the extent of the findings.
Matt Herrick, a spokesperson for the International Dairy Foods Association, said that time and temperature regulations for pasteurization ensure that the commercial U.S. milk supply is safe. Remnants of the virus “have zero impact on human health,” he wrote in an email.
Scientists confirmed the H5N1 virus in dairy cows in March after weeks of reports that cows in Texas were suffering from a mysterious malady. The cows were lethargic and saw a dramatic reduction in milk production. Although the H5N1 virus is lethal to commercial poultry, most infected cattle seem to recover within two weeks, experts said.
To date, two people in the U.S. have been infected with bird flu. A Texas dairy worker who was in close contact with an infected cow recently developed a mild eye infection and has recovered. In 2022, a prison inmate in a work program caught it while killing infected birds at a Colorado poultry farm. His only symptom was fatigue, and he recovered.




Health
Remnants of bird flu virus found in pasteurized milk, FDA says – Hamilton Spectator


/* OOVVUU Targeting */
const path = ‘/life’;
const siteName = ‘thespec.com’;
let domain = ‘thestar.com’;
if (siteName === ‘thestar.com’)
domain = ‘thestar.com’;
else if (siteName === ‘niagarafallsreview.ca’)
domain = ‘niagara_falls_review’;
else if (siteName === ‘stcatharinesstandard.ca’)
domain = ‘st_catharines_standard’;
else if (siteName === ‘thepeterboroughexaminer.com’)
domain = ‘the_peterborough_examiner’;
else if (siteName === ‘therecord.com’)
domain = ‘the_record’;
else if (siteName === ‘thespec.com’)
domain = ‘the_spec’;
else if (siteName === ‘wellandtribune.ca’)
domain = ‘welland_tribune’;
else if (siteName === ‘bramptonguardian.com’)
domain = ‘brampton_guardian’;
else if (siteName === ‘caledonenterprise.com’)
domain = ‘caledon_enterprise’;
else if (siteName === ‘cambridgetimes.ca’)
domain = ‘cambridge_times’;
else if (siteName === ‘durhamregion.com’)
domain = ‘durham_region’;
else if (siteName === ‘guelphmercury.com’)
domain = ‘guelph_mercury’;
else if (siteName === ‘insidehalton.com’)
domain = ‘inside_halton’;
else if (siteName === ‘insideottawavalley.com’)
domain = ‘inside_ottawa_valley’;
else if (siteName === ‘mississauga.com’)
domain = ‘mississauga’;
else if (siteName === ‘muskokaregion.com’)
domain = ‘muskoka_region’;
else if (siteName === ‘newhamburgindependent.ca’)
domain = ‘new_hamburg_independent’;
else if (siteName === ‘niagarathisweek.com’)
domain = ‘niagara_this_week’;
else if (siteName === ‘northbaynipissing.com’)
domain = ‘north_bay_nipissing’;
else if (siteName === ‘northumberlandnews.com’)
domain = ‘northumberland_news’;
else if (siteName === ‘orangeville.com’)
domain = ‘orangeville’;
else if (siteName === ‘ourwindsor.ca’)
domain = ‘our_windsor’;
else if (siteName === ‘parrysound.com’)
domain = ‘parrysound’;
else if (siteName === ‘simcoe.com’)
domain = ‘simcoe’;
else if (siteName === ‘theifp.ca’)
domain = ‘the_ifp’;
else if (siteName === ‘waterloochronicle.ca’)
domain = ‘waterloo_chronicle’;
else if (siteName === ‘yorkregion.com’)
domain = ‘york_region’;
let sectionTag = ”;
try
if (domain === ‘thestar.com’ && path.indexOf(‘wires/’) = 0)
sectionTag = ‘/business’;
else if (path.indexOf(‘/autos’) >= 0)
sectionTag = ‘/autos’;
else if (path.indexOf(‘/entertainment’) >= 0)
sectionTag = ‘/entertainment’;
else if (path.indexOf(‘/life’) >= 0)
sectionTag = ‘/life’;
else if (path.indexOf(‘/news’) >= 0)
sectionTag = ‘/news’;
else if (path.indexOf(‘/politics’) >= 0)
sectionTag = ‘/politics’;
else if (path.indexOf(‘/sports’) >= 0)
sectionTag = ‘/sports’;
else if (path.indexOf(‘/opinion’) >= 0)
sectionTag = ‘/opinion’;
} catch (ex)
const descriptionUrl = ‘window.location.href’;
const vid = ‘mediainfo.reference_id’;
const cmsId = ‘2665777’;
let url = `https://pubads.g.doubleclick.net/gampad/ads?iu=/58580620/$domain/video/oovvuu$sectionTag&description_url=$descriptionUrl&vid=$vid&cmsid=$cmsId&tfcd=0&npa=0&sz=640×480&ad_rule=0&gdfp_req=1&output=vast&unviewed_position_start=1&env=vp&impl=s&correlator=`;
url = url.split(‘ ‘).join(”);
window.oovvuuReplacementAdServerURL = url;
The U.S. Food and Drug Administration said Tuesday that samples of pasteurized milk had tested positive for remnants of the bird flu virus that has infected dairy cows.
The agency stressed that the material is inactivated and that the findings “do not represent actual virus that may be a risk to consumers.” Officials added that they’re continuing to study the issue.
-



 Health12 hours ago
Health12 hours agoRemnants of bird flu virus found in pasteurized milk, FDA says
-
Art18 hours ago
Mayor's youth advisory council seeks submissions for art gala – SooToday
-



 Health16 hours ago
Health16 hours agoBird flu virus found in grocery milk as officials say supply still safe
-
Media23 hours ago
Jon Stewart Slams the Media for Coverage of Trump Trial – The New York Times
-



 Investment17 hours ago
Investment17 hours agoTaxes should not wag the tail of the investment dog, but that’s what Trudeau wants
-
News17 hours ago
Peel police chief met Sri Lankan officer a court says ‘participated’ in torture – Global News
-



 Science21 hours ago
Science21 hours agoiN PHOTOS: Nature lovers celebrate flora, fauna for Earth Day in Kamloops, Okanagan | iNFOnews | Thompson-Okanagan's News Source – iNFOnews
-
Art16 hours ago
'Lost' Gustav Klimt painting to be auctioned – BBC.com






