Health
Large antibody study offers hope for virus vaccine efforts – Cochrane Today
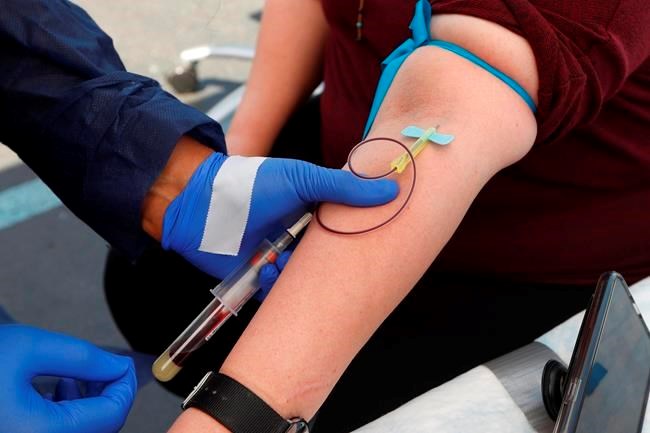
Antibodies that people make to fight the new coronavirus last for at least four months after diagnosis and do not fade quickly as some earlier reports suggested, scientists have found.
Tuesday’s report, from tests on more than 30,000 people in Iceland, is the most extensive work yet on the immune system’s response to the virus over time, and is good news for efforts to develop vaccines.
If a vaccine can spur production of long-lasting antibodies as natural infection seems to do, it gives hope that “immunity to this unpredictable and highly contagious virus may not be fleeting,” scientists from Harvard University and the U.S. National Institutes of Health wrote in a commentary published with the study in the New England Journal of Medicine.
One of the big mysteries of the pandemic is whether having had the coronavirus helps protect against future infection, and for how long. Some smaller studies previously suggested that antibodies may disappear quickly and that some people with few or no symptoms may not make many at all.
The new study was done by Reykjavik-based deCODE Genetics, a subsidiary of the U.S. biotech company Amgen, with several hospitals, universities and health officials in Iceland. The country tested 15% of its population since late February, when its first COVID-19 cases were detected, giving a solid base for comparisons.
Scientists used two different types of coronavirus testing: the kind from nose swabs or other samples that detects bits of the virus, indicating infection, and tests that measure antibodies in the blood, which can show whether someone was infected now or in the past.
Blood samples were analyzed from 30,576 people using various methods, and someone was counted as a case if at least two of the antibody tests were positive. These included a range of people, from those without symptoms to people hospitalized with signs of COVID-19.
In a subgroup who tested positive, further testing found that antibodies rose for two months after their infection initially was diagnosed and then plateaued and remained stable for four months.
Previous studies suggesting antibodies faded quickly may have been just looking at the first wave of antibodies the immune system makes in response to infection; those studies mostly looked 28 days after diagnosis. A second wave of antibodies forms after a month or two into infection, and this seems more stable and long-lasting, the researchers report.
The results don’t necessarily mean that all countries’ populations will be the same, or that every person has this sort of response. Other scientists recently documented at least two cases where people seem to have been reinfected with the coronavirus months after their first bout.
The new study also found:
— Testing through the bits-of-virus method that’s commonly done in community settings missed nearly half of people who were found to have had the virus by blood antibody testing. That means the blood tests are far more reliable and better for tracking spread of the disease in a region and for guiding decisions and returning to work or school, researchers say.
— Nearly a third of infections were in people who reported no symptoms.
— Nearly 1% of Iceland’s population was infected in this first wave of the pandemic, meaning the other 99% are still vulnerable to the virus.
— The infection fatality rate was 0.3%. That’s about three times the fatality rate of seasonal flu and in keeping with some other more recent estimates, said Dr. Derek Angus, critical care chief at the University of Pittsburgh Medical Center.
Although many studies have been reporting death rates based on specific groups such as hospitalized patients, the rate of death among all infected with the coronavirus has been unknown.
The news that natural antibodies don’t quickly disappear “will be encouraging for people working on vaccines,” Angus said.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Marilynn Marchione, The Associated Press
Health
New technology to advance women’s cancer care at Southlake


|
|
NEWS RELEASE
SOUTHLAKE REGIONAL HEALTH CENTRE
**************************
This Cancer Awareness Month, Southlake is adding advanced technologies to detect and treat breast cancer and other women’s cancers thanks to generous community donor support, most recently through the HERE is Where Cancer Meets its Match campaign. New cancer care technology, including new mammography machines, the MyoSure System and the MOLLI 2® System will make a measurable impact in diagnosing and treating women’s cancers in the communities Southlake serves.
Southlake is installing three new mammography machines to expand its breast cancer screening program to 1,500 more women each year. Two of these machines have new biopsy capabilities that will reduce the number of cancelled exams due to equipment failure, ensuring timely care for women. Women ages 40 to 49 years old will be able to self-refer for publicly funded mammograms through the Ontario Breast Screening Program starting this fall.
“Early detection is critical when treating breast cancer and other women’s cancers,” said Lorrie Reynolds, Director, Regional Cancer Program at Southlake. “We treat more than 1,700 breast cancer patients at Southlake every year. By adding advanced technology, like the new mammography machines, we’re ensuring women have the best experience at Southlake.”
Southlake is also introducing the MyoSure System, an innovative technology that can help detect female reproductive cancers. Damaged tissue in a woman’s uterus such as fibroids and polyps can now be removed in a precise, minimally invasive procedure that leaves the rest of the uterus intact. This will improve the overall patient experience by supporting faster recovery, reducing the risk of infection and giving more women the option to have children. An estimated 200 women per year will benefit from the MyoSure System.
The new mammography machines and the MyoSure System build on Southlake’s recent investment in the MOLLI 2® System, a made-in-Canada wire-free breast localization technology. This technology is considerably less invasive and more accurate when compared to wire-guided localization, resulting in a better patient experience and improved cosmetic outcomes. More than 200 women each year will benefit from this innovative medical device as they are treated for breast cancer at Southlake.
“As a clinician caring for women with cancer in our community, I’m incredibly proud of the work Southlake is doing to advance women’s health and improve patient experiences,” said Sara Temple, MD, Surgical Oncologist and Chief of Surgery at Southlake. “Women who visit Southlake can be confident that they are receiving leading edge care, close to home when they need it most.”
The World Health Organization anticipates a 77 per cent increase in cancer diagnoses by 2050. Southlake serves some of the fastest growing communities in Canada and anticipates that the number of patients requiring cancer care will grow. By investing in new technology, Southlake is ensuring that women in the communities it serves have access to leading edge cancer care. All of these investments were funded with support from community donors who generously gave to Southlake to support investments into women’s health at the hospital.
“The generosity of our donor community and the impact they have made for women receiving cancer diagnosis and treatment at Southlake is something we can all take great pride in,” said Jennifer Ritter, President and CEO of Southlake Foundation. “From our Women’s Health Initiative donors supporting new mammography machines, to the Ladies in Philanthropy for Southlake funding the MOLLI 2 System, to our long-standing partners The Edge Benefits and Pheasant Run Golf Club enabling the introduction of MyoSure System through their joint annual charity golf tournament, we are incredibly lucky to share a vision of access to exceptional care for everyone who depends on Southlake when they need us most. Thank you, to every donor who contributed to these important upgrades to care for women.”
Southlake Foundation’s HERE is Where Cancer Meets its Match campaign supports the Stronach Regional Cancer Centre at Southlake. For more information or to make a donation, visit: southlake.ca/HERE.





Health
Pasteurized milk includes remnants of H5N1 bird flu, U.S. officials say


|
|
The U.S. Food and Drug Administration says that samples of pasteurized milk have tested positive for remnants of the bird flu virus that has infected dairy cows.
The agency stressed that the material is inactivated and that the findings “do not represent actual virus that may be a risk to consumers.” Officials added that they’re continuing to study the issue.
“To date, we have seen nothing that would change our assessment that the commercial milk supply is safe,” the FDA said in a statement on Tuesday.
The announcement comes nearly a month after an avian influenza virus that has sickened millions of wild and commercial birds in recent years was detected in dairy cows in at least eight states. The Agriculture Department (USDA) says 33 herds have been affected to date.
FDA officials didn’t indicate how many samples they tested or where they were obtained. The agency has been evaluating milk during processing and from grocery stores, officials said. Results of additional tests are expected in “the next few days to weeks.”
WATCH | Bird flu spread in U.S. cows:
For the first time ever, avian influenza, or H5N1 bird flu, was detected in roughly a dozen dairy cow herds across the U.S. About That producer Lauren Bird explores why scientists and public health officials are concerned about the cross-species transmission and whether humans are now at higher risk.
The polymerase chain reaction (PCR) lab test the FDA used would have detected viral genetic material even after live virus was killed by pasteurization, or heat treatment, said Lee-Ann Jaykus, an emeritus food microbiologist and virologist at North Carolina State University
“There is no evidence to date that this is infectious virus, and the FDA is following up on that,” Jaykus said.
Officials with the FDA and the USDA had previously said milk from affected cattle did not enter the commercial supply. Milk from sick animals is supposed to be diverted and destroyed. Federal regulations require milk that enters interstate commerce to be pasteurized.
Tests for viable virus underway, agency says
Because the detection of the bird flu virus known as Type A H5N1 in dairy cattle is new and the situation is evolving, no studies on the effects of pasteurization on the virus have been completed, FDA officials said. But past research shows that pasteurization is “very likely” to inactivate heat-sensitive viruses like H5N1, the agency added.
The agency said it has been evaluating milk from affected animals, in the processing system and on the shelves. It said it is completing a large, representative national sample to understand the extent of the findings.
Matt Herrick, a spokesperson for the International Dairy Foods Association, said that time and temperature regulations for pasteurization ensure that the commercial U.S. milk supply is safe. Remnants of the virus “have zero impact on human health,” he wrote in an email.
Scientists confirmed the H5N1 virus in dairy cows in March after weeks of reports that cows in Texas were suffering from a mysterious malady. The cows were lethargic and saw a dramatic reduction in milk production. Although the H5N1 virus is lethal to commercial poultry, most infected cattle seem to recover within two weeks, experts said.
To date, two people in the U.S. have been infected with bird flu. A Texas dairy worker who was in close contact with an infected cow recently developed a mild eye infection and has recovered. In 2022, a prison inmate in a work program caught it while killing infected birds at a Colorado poultry farm. His only symptom was fatigue, and he recovered.





Health
Remnants of bird flu virus found in pasteurized milk, FDA says


|
|
The U.S. Food and Drug Administration said Tuesday that samples of pasteurized milk had tested positive for remnants of the bird flu virus that has infected dairy cows.
The agency stressed that the material is inactivated and that the findings “do not represent actual virus that may be a risk to consumers.” Officials added that they’re continuing to study the issue.





-



 Health20 hours ago
Health20 hours agoRemnants of bird flu virus found in pasteurized milk, FDA says
-
News17 hours ago
Amid concerns over ‘collateral damage’ Trudeau, Freeland defend capital gains tax change
-
Art21 hours ago
Random: We’re In Awe of Metaphor: ReFantazio’s Box Art
-
Art24 hours ago
‘Lost’ Gustav Klimt painting to be auctioned
-
Art14 hours ago
The unmissable events taking place during London’s Digital Art Week
-
Tech21 hours ago
Surprise Apple Event Hints at First New iPads in Years
-
Media20 hours ago
Vaughn Palmer: B.C. premier gives social media giants another chance
-



 Politics18 hours ago
Politics18 hours agoHow Michael Cohen and Trump went from friends to foes





