Health
Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland—A multicentre cohort study
|
|
Abstract
Background
Knowledge about protection conferred by previous Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection and/or vaccination against emerging viral variants allows clinicians, epidemiologists, and health authorities to predict and reduce the future Coronavirus Disease 2019 (COVID-19) burden. We investigated the risk and symptoms of SARS-CoV-2 (re)infection and vaccine breakthrough infection during the Delta and Omicron waves, depending on baseline immune status and subsequent vaccinations.
Methods and findings
In this prospective, multicentre cohort performed between August 2020 and March 2022, we recruited hospital employees from ten acute/nonacute healthcare networks in Eastern/Northern Switzerland. We determined immune status in September 2021 based on serology and previous SARS-CoV-2 infections/vaccinations: Group N (no immunity); Group V (twice vaccinated, uninfected); Group I (infected, unvaccinated); Group H (hybrid: infected and ≥1 vaccination). Date and symptoms of (re)infections and subsequent (booster) vaccinations were recorded until March 2022. We compared the time to positive SARS-CoV-2 swab and number of symptoms according to immune status, viral variant (i.e., Delta-dominant before December 27, 2021; Omicron-dominant on/after this date), and subsequent vaccinations, adjusting for exposure/behavior variables.
Among 2,595 participants (median follow-up 171 days), we observed 764 (29%) (re)infections, thereof 591 during the Omicron period. Compared to group N, the hazard ratio (HR) for (re)infection was 0.33 (95% confidence interval [CI] 0.22 to 0.50, p < 0.001) for V, 0.25 (95% CI 0.11 to 0.57, p = 0.001) for I, and 0.04 (95% CI 0.02 to 0.10, p < 0.001) for H in the Delta period. HRs substantially increased during the Omicron period for all groups; in multivariable analyses, only belonging to group H was associated with protection (adjusted HR [aHR] 0.52, 95% CI 0.35 to 0.77, p = 0.001); booster vaccination was associated with reduction of breakthrough infection risk in groups V (aHR 0.68, 95% CI 0.54 to 0.85, p = 0.001) and H (aHR 0.67, 95% CI 0.45 to 1.00, p = 0.048), largely observed in the early Omicron period. Group H (versus N, risk ratio (RR) 0.80, 95% CI 0.66 to 0.97, p = 0.021) and participants with booster vaccination (versus nonboosted, RR 0.79, 95% CI 0.71 to 0.88, p < 0.001) reported less symptoms during infection. Important limitations are that SARS-CoV-2 swab results were self-reported and that results on viral variants were inferred from the predominating strain circulating in the community at that time, rather than sequencing.
Conclusions
Our data suggest that hybrid immunity and booster vaccination are associated with a reduced risk and reduced symptom number of SARS-CoV-2 infection during Delta- and Omicron-dominant periods. For previously noninfected individuals, booster vaccination might reduce the risk of symptomatic Omicron infection, although this benefit seems to wane over time.
Author summary
Why was this study done?
- Preexisting immunity against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)—either from previous infection or vaccination—confers protection against severe Coronavirus Disease 2019 (COVID-19).
- Few studies have prospectively determined the SARS-CoV-2 infection risk based on large-scale serologic testing to detect previous asymptomatic infections.
- Real-world evaluations of infections during periods with distinct SARS-CoV-2 variants are valuable to assess protection by preexisting immunity and inform health policy guidelines.
What did the researchers do and find?
- In September 2021, 2,554 healthcare workers were classified into four different groups based on previous SARS-CoV-2 serology results and infection/vaccination history.
- Participants were followed until March 2022 to assess the association of immune status and additional vaccinations with self-reported COVID-19 and symptoms.
- Hybrid immunity (i.e., previous infection and at least one vaccination) resulted in a reduced SARS-CoV-2 infection risk and less symptoms during the Delta or Omicron period.
- Booster vaccination was associated with reduced infection risk and less symptoms during the first half of the Omicron period analysed in our study.
What do these findings mean?
- Individuals who were previously infected and vaccinated seem to be best protected and exhibit less symptoms of SARS-CoV-2 infection than those with other immune status.
- Booster vaccination might further reduce both the risk of Omicron breakthrough infection and the number of reported symptoms, although this benefit fades over time.
- These findings might inform healthcare providers and public health authorities in estimating the risk of SARS-CoV-2 (re)infection in individuals or communities.
Citation: Babouee Flury B, Güsewell S, Egger T, Leal O, Brucher A, Lemmenmeier E, et al. (2022) Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland—A multicentre cohort study. PLoS Med 19(11):
e1004125.
https://doi.org/10.1371/journal.pmed.1004125
Academic Editor: James G. Beeson, Burnet Institute, AUSTRALIA
Received: May 31, 2022; Accepted: October 14, 2022; Published: November 7, 2022
Copyright: © 2022 Babouee Flury et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data are available at https://dx.doi.org/10.5281/zenodo.7149075.
Funding: This work was supported by the Swiss National Sciences Foundation (grant number 31CA30_196544 to PK and CRK; grant number PZ00P3_179919 to PK), the Federal Office of Public Health (grant number 20.008218/421-28/1), and the Health Department of the Canton of St. Gallen. Funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations:
aHR,
adjusted HR; anti-N,
anti-nucleocapsid; anti-S,
anti-spike; aRR,
adjusted rate ratio; CI,
confidence interval; COVID-19,
Coronavirus Disease 2019; HCW,
healthcare worker; HR,
hazard ratio; NPS,
nasopharyngeal swab; PCR,
polymerase chain reaction; RAD,
rapid antigen diagnostic; RR,
risk ratio; SARS-CoV-2,
Severe Acute Respiratory Syndrome Coronavirus 2
Background
Mitigation of Coronavirus Disease 2019 (COVID-19) relies on establishing an ideally long-lasting immune barrier against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Studies from the pre-Omicron era show that specific neutralizing antibodies as marker for humoral immunity reduce the risk of symptomatic (re)infection and thus disease recurrence upon reexposure to a homologous viral strain [1]. Although natural infection with SARS-CoV-2 elicits a broader humoral response than mRNA vaccine administration, levels of neutralizing antibodies are lower after natural infection [2]. Concerning (re)infection however, previous infection is associated with 85% protection over at least 9 months [3]; also, infected individuals might be even better protected than only vaccinated individuals [4]. A still lower reinfection risk has been reported for individuals with hybrid immunity, i.e., SARS-CoV-2 infection plus at least one vaccination [5–10].
Yet, weak or waning humoral response in tandem with the emergence of new viral variants, capable of potentially escaping immune response, lead to a continued risk of reinfection with heterologous SARS-CoV-2 strains [1,11,12]. This is particularly true for the Omicron variant [13], which is the predominant viral strain globally as of October 2022 [14], causing mostly milder infections compared to preceding strains [15–17]. Available evidence from adult and pediatric populations points towards good effectiveness of prior mRNA vaccination against severe COVID-19 and hospitalisation due to Omicron variants, but less so against asymptomatic and symptomatic, mild breakthrough infections [11,18–21]. Similar to the pre-Omicron era, some studies have shown that previous infection or hybrid immunity (compared to vaccination alone) might provide better protection against the Omicron variant. However, these studies were either small [22] or relied on population-based data [23], ignoring the fact that a substantial proportion of SARS-CoV-2 infections remain undetected with the risk of misclassification [24]. Also, behavior and exposure variables, which are likely to be different between previously vaccinated and unvaccinated individuals, were not considered in these studies. The influence of type and timing of SARS-CoV-2 vaccination on eventual risk of breakthrough infection has been described for previous variants, but not for Omicron [7,25].
Within a prospective, multicentre healthcare worker (HCW) cohort study, we aimed to determine the risk for and symptoms of COVID-19 by comparing Delta- and Omicron-dominant periods, depending on previous immune status based on infection/vaccination history and serology results. Furthermore, we examined the role of booster vaccination on these outcomes. For those with two vaccinations, we assessed the role of mRNA vaccine type and timing of vaccine doses on breakthrough infection risk.
Methods
Study design and population
This prospective cohort (SURPRISE) was initiated in summer 2020, after the first COVID-19 wave in Switzerland. The study was approved by the ethics committee of Eastern Switzerland (#2020–00502), and participants provided digital written informed consent. Health professionals (with and without patient contact) aged 16 years or older from ten healthcare networks located in Northern and Eastern Switzerland were included between June 2020 and March 2021. From their inclusion until September 2021, participants were prospectively followed through weekly questionnaires on SARS-CoV-2 infections/vaccinations, and periodic SARS-CoV-2 serology measurements in August 2020, January 2021, and August/September 2021 [26]. Participants without available serology from August/September 2021 were excluded. For the present work, no prespecified analysis plan was designed.
Baseline immune status was assessed as of September 20, 2021 (Fig 1), based on previous infection/vaccination history and all available serology results. During the local emergence of the Delta (October to December 2021) and Omicron B.1.1.529.1 (Nextstrain 21K; BA.1) variant (January to March 2022), the follow-up survey through questionnaires was continued at monthly intervals. Information collected included SARS-CoV-2 exposures, reports of nasopharyngeal swab (NPS) tests (positive and negative), symptoms associated with positive NPS, and receipt of subsequent (booster or first) vaccinations.
SARS-CoV-2 diagnostics
Participants were asked to get tested for SARS-CoV-2 in case of compatible symptoms, according to national recommendations. SARS-CoV-2 was detected by polymerase chain reaction (PCR) or rapid antigen diagnostic (RAD) test, depending on the participating institutions. Some facilities also switched from PCR to RAD in the course. No sequencing for determination of the viral variants was performed; the viral variant was inferred from the predominating strain circulating in the community at that time (see below). To verify the completeness and accuracy of self-reported NPS results (PCR or RAD), all positives and a random sample of negatives were validated for a subgroup of HCWs from the largest participating institution as described previously [27]. Anti-nucleocapsid (anti-N) and anti-spike (anti-S) antibodies were measured using the Roche Elecsys (Roche Diagnostics, Rotkreuz, Switzerland) electro-chemiluminescence immunoassay [28].
Definition of predictor variables
We defined four distinct immune status groups (i.e., main predictor) as of September 20, 2021, according to previous questionnaires and serology results: (i) Group N (reference): no reported infection and anti-N/anti-S negative and no previous SARS-CoV-2 vaccination; (ii) Group V (vaccinated): no reported infection and anti-N negative, but twice vaccinated (with any time interval between doses) with the second dose being at least 7 days ago; (iii) Group I (infected): infection reported or anti-N positive (at any time), but no vaccination; (iv) Group H (hybrid immunity): reported infection or anti-N positive (at any time) and vaccination (≥1 dose) at least 7 days ago. In addition, we collected information on vaccination after September 20, 2021, either booster vaccination (available from November 2021 on) for groups V and H or first vaccinations for groups N and I. Again, participants were considered as boosted 7 days after receipt of the vaccine. Immune status was treated as time-dependent variable, so that participants switched from group I to H after the first vaccination, and from group N to V after the second vaccination. Participants in group N with only one vaccination were not considered and were only included 7 days after receipt of their second dose. The different groups and outcomes are illustrated in S1 Fig. For other predictor variables, S1 Table shows definitions and time points of the corresponding questionnaire.
Outcomes
The main outcome was time to the first SARS-CoV-2-positive NPS reported after September 20, 2021. Outcomes occurring between September 20, 2021 and March 6, 2022 were included. The period before December 27, 2021 was defined as Delta-dominant (i.e., Delta period), the period on and after this date as Omicron-dominant (i.e., Omicron period), based on sequencing data from North-Eastern Switzerland [29]. We treated our outcome as a survival event, i.e., participants were no longer at risk after their first positive NPS. We also calculated the number of symptoms reported during SARS-CoV-2 infection.
Statistical analysis regarding infection risk
We computed Kaplan–Meier curves to compare the occurrence of SARS-CoV-2 (re)infection (first positive NPS) through time according to immune status; noninfected participants were censored at the time of the last available follow-up questionnaire. The risk of (re)infection was compared among groups (treated as time-dependent variable) using Cox regression; hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated. This analysis was performed separately for the Delta and Omicron periods, with and without adjusting for receipt of booster vaccination. In addition, Kaplan–Meier curves were computed to visualize the association of booster vaccination on infection risk during the Omicron period, when most of these vaccinations had already been received.
Multivariable Cox-regression analysis was performed to correct for additional confounding variables, which were a priori selected based on previous analyses and expected importance [30]. Multiple imputation was used to substitute missing values (S1 Methods). Time-dependent variables were, besides immune status, receipt of booster vaccination, SARS-CoV-2 infection of a household contact within the same month, and documentation of ≥1 negative test in the previous month (to adjust for differences in testing behaviour). Time-independent variables included baseline anthropometric data and further variables reflecting SARS-CoV-2 risk exposures and behaviours (S1 Table). We performed a sensitivity analysis excluding infections occurring during the period of variant overlap between December 6, 2021 and January 3, 2022 to minimize contamination of infections occurring in the Delta and Omicron periods with the respective other viral variant.
Supplementary analyses
Because model diagnostics showed that the influence of booster vaccination was time dependent in the Omicron period, we further split this period into an early (before February 15, 2022) and late Omicron phase (after this date).
To estimate the influence of time since last immunization event, we included time from previous infection or vaccination (i.e., time of preimmunization) until September 20, 2021 for those individuals where this information was available. The exact day of initial infection could not be assessed in those with only positive anti-N but no report of positive NPS; also, group N, which per definition did not have an immunization event, was excluded and group V was chosen as reference category instead.
Finally, to assess the impact of type and timing of vaccination within group V, we included the type of vaccine (i.e., mRNA-1273 or BNT162b2), the time of preimmunization, and time between first and second vaccination dose.
Frequency of SARS-CoV-2 symptoms
We used univariable and multivariable Poisson regression to assess the impact of baseline immune status on symptom number for infections that occurred before any booster or first vaccination. Covariables included the virus variants (Delta versus Omicron period), the month of (re)infection (to adjust for the fact that immune protection wanes over time), and a priori selected variables based on their importance in previous analyses [31].
To assess the impact of booster vaccination on number of symptoms, we performed a second analysis restricted to groups V and H, and to infections occurring during the Omicron period (because of the small number of infections preceded by booster in the Delta period). The study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (S1 STROBE Checklist) [32]. R version 4.1.2 was used for statistical analyses.
Results
Study population
Of the 5,792 initial cohort participants, 2,595 (45%) underwent serology testing in August/September 2021 and completed at least one follow-up questionnaire. Thereof, 2,554 were classified into one of the immune status groups at baseline: 581 (22.7%) in group H, 162 (6.3%) in I, 1,643 (64.3%) in V, and 168 (6.6%) in N; additionally, 41 individuals were assigned to group V during follow-up (after their second vaccination) (S2 Fig). Median follow-up was 171 days (interquartile range 131 to 171). Baseline characteristics are summarized in Table 1.
SARS-CoV-2 (re)infections by immune status and time period
A total of 764 (29.4%) infections were reported in 2,595 participants, whereof 173 (22.6%) occurred during the Delta and 591 (77.4%) during the Omicron period. During Delta, the risk for COVID-19 was significantly reduced for groups V (HR 0.33, 95% CI 0.22 to 0.50, p < 0.001), I (HR 0.25, 95% CI 0.11 to 0.57, p = 0.001), and H (HR 0.04, 95% CI 0.02 to 0.10, p < 0.001) compared to group N. These associations were less pronounced during the Omicron period for all groups (Fig 2).
Left: influence of baseline immune status on the time course of (re)infection events, shown separately for the two periods (Delta vs. Omicron) by resetting Kaplan–Meier curves to 0 on December 27, 2021. Note that group differences depicted in the graph include any impact of booster vaccination (in groups V and H). Right: HRs with 95% CIs from Cox regression regarding risk of SARS-CoV-2 (re)infection for each immune status compared with group N (no previous infection or vaccination), both without and with adjustment for booster vaccination. CI, confidence interval; HR, hazard ratio; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Booster vaccination and risk of infection
By March 2022, 80% of participants in groups V and H had received a booster (median time of booster was December 14, 2021) (S3 Fig). Adjusting the univariable model for booster vaccination, the adjusted HR (aHR) remained similar for the Delta period as in the unadjusted model. In the Omicron period, only group H showed a reduced risk (aHR 0.45, 0.30 to 0.67, p < 0.001) compared to group N, whereas no significant risk reduction was observed for groups V and I (Fig 2). Restricting the analysis to the Omicron period, receipt of booster vaccination was associated with reduced infection risk in groups V (aHR 0.68, 95% CI 0.54 to 0.85, p = 0.001) and H (aHR 0.67, 95% CI 0.45 to 1.00, p = 0.048) (Fig 3).
Note that participants receiving their booster after December 27, 2021 were initially classified as “no” and subsequently switched to “yes,” so that numbers at risk for “yes” increase initially. CI, confidence interval; HR, hazard ratio.
Multivariable analysis and sensitivity analyses
When models included exposure and behaviour variables, results were similar, with group H being the only group with reduced infection risk (aHR 0.52, 95% CI 0.35 to 0.77, p = 0.001) in the Omicron period (Table 2). The main risk correlate for SARS-CoV-2 positivity was having a SARS-CoV-2-positive household (as reported by the participant), both in the Delta (aHR 9.66, 95% CI 7.15 to 13.05, p < 0.001) and the Omicron period (aHR 6.17, 95% CI 5.24 to 7.27, p < 0.001).
Excluding infections during the Delta-Omicron overlap period and performing further missing value imputations as well as a complete case analysis yielded similar HRs as the main analysis (S3 Table).
Supplementary analyses
Splitting the Omicron period into an early and late phase suggested a benefit of the booster vaccination for the early Omicron phase (aHR 0.60, 95% CI 0.47 to 0.76, p < 0.001), but not for the late Omicron phase (aHR 1.02, 95% CI 0.75 to 1.38, p = 0.90) (S4 Table).
Time since preimmunization was associated with infection risk during the Delta period (aHR 1.16 per additional months, 95% CI 1.06 to 1.28, p = 0.003), but not the Omicron period (aHR 1.00, 95% CI 0.96 to 1.04, p = 0.82). Hybrid immunity remained the immune status, which best protected against infection in both periods. Of note, group I (compared to group V) was better protected against infection in the Delta period (aHR 0.27, 95% CI 0.09 to 0.82, p = 0.023), but not in the Omicron period (aHR 0.84, 95% CI 0.49 to 1.44, p = 0.527) (S5 Table).
Within group V, neither the time interval between dose 1 and dose 2 (HR 0.96 per month, 95% CI 0.73 to 1.27 p = 0.788) nor receipt of the mRNA-1273 (versus BNT162b2) vaccine (HR 0.79, 95% CI 0.62 to 1.01, p = 0.060) were significantly associated with reduced infection risk (S6 Table).
SARS-CoV-2 symptoms according to immune status and time period
Participants reported a median of four symptoms during SARS-CoV-2 episodes in the Delta period, and three in the Omicron period (Fig 4). Group H reported fewer symptoms (median of three) than group N (median of four, adjusted rate ratio [aRR] 0.81, 95% CI 0.67 to 0.97, p = 0.026), even after adjusting for multiple covariables. Groups V and I did not report less symptoms compared to group N (S7 Table). Restricting the analysis to vaccinated participants, hybrid immunity (group H versus V, aRR 0.80, 95% CI 0.71 to 0.91, p < 0.001) and receipt of booster vaccination (aRR 0.79, 95% CI 0.71 to 0.88, p < 0.001), were associated with fewer symptoms, whereas having a comorbidity at baseline (aRR 1.17, 95% CI 1.06 to 1.28, p = 0.001) and being infected later during the Omicron period (aRR 1.22 per additional months, 95% CI 1.13 to 1.31, p < 0.001) were associated with more symptoms (S8 Table).
Number of symptoms reported after (re)infection by baseline immune status, grouped by Delta and Omicron periods (panel A, left) and receipt of booster (panel B, right). N (no immunity): no reported infection and anti-N/-S negative and no previous SARS-CoV-2 vaccination; V (vaccinated): no reported infection and anti-N negative, but twice vaccinated; I (infected): infection reported or anti-N positive (at any time), but no vaccination; H (hybrid immunity): reported infection or anti-N positive (at any time) and vaccination (≥1 dose).
Discussion
In this prospective multicentre study, we observed that participants with hybrid immunity and those receiving booster vaccination reported the lowest risk for COVID-19 during the Delta and Omicron periods, and, when infected during the Omicron period, were less symptomatic than those only vaccinated or previously infected. However, this association with booster vaccination waned over time. Type and timing of baseline mRNA vaccines were not associated with the outcomes.
Hybrid immunity, defined as immunity acquired from previous infection plus at least one vaccination, was shown to be associated with reduced risk of SARS-CoV-2 reinfection compared to previous infection only, for up to 9 months [33]. We confirm findings of this Swedish study, which ended in October 2021 (i.e., before Delta was the predominant variant in Europe including Sweden) [34]. Also, our data are in line with a study from Israel performed mainly during the Delta period showing that persons with hybrid immunity were better protected against breakthrough infection compared to only vaccinated persons [10]. In addition, our findings suggest that hybrid immunity acquired from infections with previous variants not only provides protection against infections by the Delta, but also the Omicron variant, as described in a previous study from Qatar [23]. In contrast to this latter study, which relied on population-level data, we used baseline serology results allowing us to additionally capture previous asymptomatic infections and to adequately assign participants to the respective immune status groups. Furthermore, our study revealed that hybrid immunity was the only immune status associated with less symptoms compared to the group without any preexisting immunity, for both time periods. These findings are in line with results of a laboratory-based study, which showed that antibodies from sera of people with hybrid immunity were able to better neutralize the Omicron variant, compared to antibodies from only vaccinated or infected individuals [35].
When ignoring the booster, we did no longer observe any additional protection of two-dose vaccination in noninfected participants during the Omicron period. This finding adds to data from Qatar, where the effectiveness of two-dose BNT162b2 vaccination against symptomatic Omicron infection was found to be negligible [23]. For the Delta period, we observed a higher risk of (re)infection in twice vaccinated compared to previously infected, nonvaccinated participants. Similarly, in a large retrospective observational study in 124,500 participants, naturally acquired immunity to SARS-CoV-2 conferred stronger protection against infection and symptomatic disease caused by the Delta variant, compared to the BNT162b2 two-dose vaccine-induced immunity [36]. The benefit of previous infection could be explained by the broader immune response elicited by natural infection, with humoral and cellular immune responses not only targeting the spike protein but also other viral antigens. However, in our study, this association was no longer apparent during the Omicron period, where the infection risk of participants with only infection-induced immunity compared to those with vaccine-induced immunity was similar.
The effectiveness of previous infection in preventing reinfection with the Alpha, Beta, and Delta variants of SARS-CoV-2 was around 90% and 60% for Omicron in another study from Qatar [37]. These estimates are higher than in our study, where previous infection was associated with a 75% risk reduction during the Delta, and 25% during the Omicron dominating period. In contrast to Altarawneh and colleagues, we included individuals with previous asymptomatic infection. As the humoral immune response elicited by asymptomatic is weaker compared to symptomatic infection [38], the benefit against reinfections might be lower, which could explain the observed discrepancy.
Previous data have shown that the effectiveness of mRNA-1273 might be superior to BNT162b2, likely because of the slower rise and faster decay of neutralizing antibody titers elicited by BNT162b2 [39,40]. Our study was not designed to assess true vaccine effectiveness. However, when adjusting for receipt of booster vaccine, the risk for breakthrough infection in participants receiving either vaccine was similar, as has also been reported from Qatar [23]; the time interval between first and second vaccine did not impact these results, as has shown previously for the ChAdOx1 vaccine [25].
Our data point towards a benefit of booster vaccination against infection in the Omicron period of approximately 30%, which is below the 47% booster effectiveness reported previously [6]. Also, in a study among US adults with COVID-like illness, the odds ratio for boosted versus nonboosted individuals was 0.16 for Delta and 0.34 for Omicron infections [19], which is in the range of a prospective cohort of frontline workers, where booster mRNA vaccine provided around 90% protection against Delta and 60% against Omicron infection [18]. In the latter study, participants were routinely tested for SARS-CoV-2, resulting in a relevant proportion of asymptomatic infections, which could have overestimated the benefit of the booster vaccination. Another explanation for the lower figures observed in our study is that we collected data over a longer period of Omicron activity (over 2 months), during which the booster effect might have waned [41]. Indeed, most participants in our cohort received their booster vaccination before onset of the Omicron period; at the same time, in a supplementary analysis of our data, the benefit associated with booster vaccination vanished in the late Omicron period.
Nevertheless, both with the Delta and the Omicron variants, mRNA booster lead to strong protection against COVID-19–related hospitalization and death [20,42]. Although these outcomes were not relevant in our context, we observed a reduction of symptoms among boosted versus nonboosted individuals during the Omicron period. However, we did not assess whether this reduction of symptoms led to less work absenteeism or visits to healthcare providers.
The most important strengths of this study are the availability of baseline serology data along with behavioural and exposure variables, the prospective nature, and the coverage of symptoms associated with COVID-19 are. Limitations of our study include that SARS-CoV-2 testing was not mandatory and that results were self-reported. However, we previously showed that self-reported NPS results were highly consistent with documented (for positive NPS) and nondocumented (negative NPS) infections [27]. In addition, we adjusted our analysis for the participants’ testing behaviour. Viral variants were categorized based on the community epidemiology only (predominating viral strain) and not on sequencing results. This potential imprecision could have led to underestimation of the differential impact of viral variant; yet, excluding infections occurring during the overlap period as sensitivity analysis did not significantly change the results. SARS-CoV-2-specific T-cell immunity is also likely relevant, for assessing the risk for (re)infection, particularly of severe infection. However, as we did not sample cells from the blood, we were not able to assess this part of the specific immune response. Our results are insofar not generalizable, as the cohort consisted of a well-defined group of young and healthy HCW with an increased exposure to SARS-CoV-2.
Conclusions
In this real-life study using large-scale serology data, we evaluated SARS-CoV-2 infections during the Delta and Omicron waves in Switzerland and observed that hybrid immunity in HCW—compared to other immune status—was associated with the lowest risk of (re)infection and less symptoms in case of infection. Booster vaccination was associated with a risk reduction and with fewer symptoms of SARS-CoV-2 breakthrough infection, although this benefit seemed to fade during the Omicron period. Thus, our findings might inform healthcare providers and public health authorities in prioritizing SARS-CoV-2 vaccinations.
Supporting information
S3 Table. Sensitivity analyses: Comparison of hazard ratios obtained in Cox models with three independent missing value imputations, without missing value imputation (i.e., complete case analysis), and with exclusion of events occurring during the period of variant overlap between December 6, 2021 and January 3, 2022.
https://doi.org/10.1371/journal.pmed.1004125.s005
(PDF)
S5 Table. Adjusted hazard ratios (HR) with 95% confidence intervals (CI) from multivariable Cox regression regarding risk of SARS-CoV-2 (re)infection; group N (no immunity) excluded and group V (vaccinated) defined as reference group.
Model additionally includes time from preimmunization to serology (i.e., months since last infection or vaccination) compared to the main analysis.
https://doi.org/10.1371/journal.pmed.1004125.s007
(PDF)
S6 Table. Hazard ratios (HR) with 95% confidence intervals (CI) from multivariable Cox regression regarding risk of SARS-CoV-2 infection in the subgroup of those vaccinated but not infected (group V).
Model includes type of vaccine and timing of vaccinations between dose 1 and dose 2.
https://doi.org/10.1371/journal.pmed.1004125.s008
(PDF)
S7 Table. Rate ratio (RR) and 95% confidence intervals (CI) from multivariable Poisson regression regarding number of symptoms reported from SARS-CoV-2 infections during the Delta and Omicron period.
Model includes only infections not preceded by booster or first vaccination.
https://doi.org/10.1371/journal.pmed.1004125.s009
(PDF)
S8 Table. Rate ratio (RR) and 95% confidence intervals (CI) from multivariable Poisson regression regarding number of symptoms reported from SARS-CoV-2 infections during the Omicron period.
Model includes booster vaccine and is therefore restricted to groups V and H.
https://doi.org/10.1371/journal.pmed.1004125.s010
(PDF)
S2 Fig. Flow sheet of participants in the SURPRISE study showing reasons (and respective number of participants) for exclusion from current analysis as well as participants within each immune status including number of subsequently vaccinated individuals, respectively.
https://doi.org/10.1371/journal.pmed.1004125.s012
(PDF)
S3 Fig. Time course of subsequent vaccinations (booster and new vaccinations).
N (no immunity): no reported infection and anti-N/-S negative and no previous SARS-CoV-2 vaccination; V (vaccinated): no reported infection and anti-N negative, but twice vaccinated; I (infected): infection reported or anti-N positive (at any time), but no vaccination; H (hybrid immunity): reported infection or anti-N positive (at any time) and vaccination (≥1 dose).
https://doi.org/10.1371/journal.pmed.1004125.s013
(TIFF)
Acknowledgments
The members of the SURPRISE study team are (in alphabetical order): Ulrike Besold, Angela Brucher, Thomas Egger, Andrée Friedl, Fabian Grässli, Sabine Güsewell, Eva Lemmenmeier, Christian R. Kahlert, Joelle Keller, Dorette Meier Kleeb, Philipp Kohler, Stefan P. Kuster, Onicio Leal, Dorette Meier Kleeb, Allison McGeer, J. Carsten Möller, Maja F. Müller, Vaxhid Musa, Manuela Ortner, Philip Rieder, Lorenz Risch, Markus Ruetti, Matthias Schlegel, Hans-Ruedi Schmid, Reto Stocker, Pietro Vernazza, Matthias von Kietzell, Danielle Vuichard-Gysin, and Benedikt Wiggli.
We would like to thank the employees of the participating healthcare institutions who either took part in this study themselves or supported it. Furthermore, we thank the laboratory staff for shipment, handling, and analysis of the blood samples.
References
- 1.
Zuiani A, Wesemann DR. Antibody Dynamics and Durability in Coronavirus Disease-19. Clin Lab Med. 2022;42:85–96. pmid:35153050 - 2.
Fraley E, LeMaster C, Geanes E, Banerjee D, Khanal S, Grundberg E, et al. Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021;19:169. pmid:34304742 - 3.
Ridgway JP, Tideman S, Wright B, Robicsek A. Rates of COVID-19 Among Unvaccinated Adults With Prior COVID-19. JAMA Netw Open. 2022;5:e227650. pmid:35442459 - 4.
Rahman S, Rahman MM, Miah M, Begum MN, Sarmin M, Mahfuz M, et al. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci Rep. 2022;12:1438. pmid:35082344 - 5.
Nordström P, Ballin M, Nordström A. Association Between Risk of COVID-19 Infection in Nonimmune Individuals and COVID-19 Immunity in Their Family Members. JAMA Intern Med. 2021;181:1589–1595. pmid:34633407 - 6.
Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Association of Prior SARS-CoV-2 Infection With Risk of Breakthrough Infection Following mRNA Vaccination in Qatar. JAMA. 2021;326:1930. pmid:34724027 - 7.
Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022;386:1207–1220. pmid:35172051 - 8.
Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta K-D, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. pmid:34650248 - 9.
Stamatatos L, Czartoski J, Wan Y-H, Homad LJ, Rubin V, Glantz H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372:1413–1418. pmid:33766944 - 10.
Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N Engl J Med. 2022;386:2201–2212. pmid:35613036 - 11.
Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386:1532–1546. pmid:35249272 - 12.
Michlmayr D, Hansen CH, Gubbels SM, Valentiner-Branth P, Bager P, Obel N, et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: A Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg Health—Eur. 2022;20:100452. pmid:35791335 - 13.
Hu M, Lin H, Wang J, Xu C, Tatem AJ, Meng B, et al. The risk of COVID-19 transmission in train passengers: an epidemiological and modelling study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020. pmid:32726405 - 14.
CoVariants. Overview of Variants in Countries. [cited 2022 Oct 6]. Available from: https://covariants.org/per-country?region=World - 15.
Misra A, Theel ES. Immunity to SARS-CoV-2: What Do We Know and Should We Be Testing for It? Humphries RM, editor. J Clin Microbiol. 2022:e00482–e00421. pmid:35249377 - 16.
Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. JAMA. 2022;327:583–584. pmid:34967859 - 17.
Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. pmid:35397851 - 18.
Yoon SK, Hegmann KT, Thiese MS, Burgess JL, Ellingson K, Lutrick K, et al. Protection with a Third Dose of mRNA Vaccine against SARS-CoV-2 Variants in Frontline Workers. N Engl J Med. 2022;386:1855–1857. pmid:35385628 - 19.
Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA. 2022. pmid:35060999 - 20.
Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Epidemiology; 2022 Mar. pmid:35654888 - 21.
Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N Engl J Med. 2022;387:525–532. pmid:35857701 - 22.
Nunes MC, Mbotwe-Sibanda S, Baillie VL, Kwatra G, Aguas R, Madhi SA. SARS-CoV-2 Omicron Symptomatic Infections in Previously Infected or Vaccinated South African Healthcare Workers. Vaccine. 2022;10:459. pmid:35335091 - 23.
Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N Engl J Med. 2022;387:21–34. pmid:35704396 - 24.
Sah P, Fitzpatrick MC, Zimmer CF, Abdollahi E, Juden-Kelly L, Moghadas SM, et al. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc Natl Acad Sci. 2021;118:e2109229118. pmid:34376550 - 25.
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet Lond Engl. 2021;397:881–891. pmid:33617777 - 26.
Kahlert CR, Persi R, Güsewell S, Egger T, Leal-Neto OB, Sumer J, et al. Non-occupational and occupational factors associated with specific SARS-CoV-2 antibodies among hospital workers–A multicentre cross-sectional study. Clin Microbiol Infect. 2021:S1198743X21002366. pmid:34020033 - 27.
Kohler P, Güsewell S, Seneghini M, Egger T, Leal O, Brucher A, et al. Impact of baseline SARS-CoV-2 antibody status on syndromic surveillance and the risk of subsequent COVID-19—a prospective multicenter cohort study. BMC Med. 2021;19:270. pmid:34649585 - 28.
Baron RC, Risch L, Weber M, Thiel S, Grossmann K, Wohlwend N, et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: a population-based study. Clin Chem Lab Med. 2020;58:2131–2140. pmid:32866113 - 29.
Overview of Variants in Countries. In: Overview of Variants in Countries [Internet]. 2022 May 6 [cited 2022 May 6]. Available from: https://covariants.org/per-country?region=Switzerland - 30.
Kahlert CR, Persi R, Güsewell S, Egger T, Leal-Neto OB, Sumer J, et al. Non-occupational and occupational factors associated with specific SARS-CoV-2 antibodies among hospital workers—A multicentre cross-sectional study. Clin Microbiol Infect. 2021;27:1336–1344. pmid:34020033 - 31.
Strahm C, Seneghini M, Güsewell S, Egger T, Leal O, Brucher A, et al. Symptoms compatible with long-COVID in healthcare workers with and without SARS-CoV-2 infection—results of a prospective multicenter cohort. Clin Infect Dis. 2022:ciac054. pmid:35090015 - 32.
STROBE, Strengthening the reporting of observational studies in epidemiology. Available from: https://www.strobe-statement.org - 33.
Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;S1473-3099(22):00143–00148. pmid:35366962 - 34.
COVID-19 Country overview report: week 17 2022. In: COVID-19 country overviews [Internet]. [cited 2022 May 5]. Available from: https://covid19-country-overviews.ecdc.europa.eu/countries/Sweden.html - 35.
Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N Engl J Med. 2022;386:698–700. pmid:35021005 - 36.
Gazit S, Shlezinger R, Perez G, Lotan R, Peretz A, Ben-Tov A, et al. SARS-CoV-2 Naturally Acquired Immunity vs. Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: a Retrospective Cohort Study. Clin Infect Dis. 2022:ciac262. pmid:35380632 - 37.
Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N Engl J Med. 2022;386:1288–1290. pmid:35139269 - 38.
Lazor-Blanchet C, Zygoura P, Dafni U, Lamoth F, Tsourti Z, Lobritz MA, et al. Low neutralizing antibody titers after asymptomatic or non-severe SARS-CoV-2 infection over a 6-month assessment period. J Inf Secur. 2022;84:722–746. pmid:35134464 - 39.
Keshavarz B, Richards NE, Workman LJ, Patel J, Muehling LM, Canderan G, et al. Trajectory of IgG to SARS-CoV-2 After Vaccination With BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison With Natural Infection. Front Immunol. 2022;13:850987. pmid:35386716 - 40.
Abu-Raddad LJ, Chemaitelly H, Bertollini R. Effectiveness of mRNA-1273 and BNT162b2 Vaccines in Qatar. N Engl J Med. 2022;386:799–800. pmid:35045222 - 41.
Patalon T, Saciuk Y, Peretz A, Perez G, Lurie Y, Maor Y, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13:3203. pmid:35680872 - 42.
Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N Engl J Med. 2022:NEJMoa2200797. pmid:35263534
Health
Toronto reports 2 more measles cases. Use our tool to check the spread in Canada – Toronto Star

/* OOVVUU Targeting */
const path = ‘/news/canada’;
const siteName = ‘thestar.com’;
let domain = ‘thestar.com’;
if (siteName === ‘thestar.com’)
domain = ‘thestar.com’;
else if (siteName === ‘niagarafallsreview.ca’)
domain = ‘niagara_falls_review’;
else if (siteName === ‘stcatharinesstandard.ca’)
domain = ‘st_catharines_standard’;
else if (siteName === ‘thepeterboroughexaminer.com’)
domain = ‘the_peterborough_examiner’;
else if (siteName === ‘therecord.com’)
domain = ‘the_record’;
else if (siteName === ‘thespec.com’)
domain = ‘the_spec’;
else if (siteName === ‘wellandtribune.ca’)
domain = ‘welland_tribune’;
else if (siteName === ‘bramptonguardian.com’)
domain = ‘brampton_guardian’;
else if (siteName === ‘caledonenterprise.com’)
domain = ‘caledon_enterprise’;
else if (siteName === ‘cambridgetimes.ca’)
domain = ‘cambridge_times’;
else if (siteName === ‘durhamregion.com’)
domain = ‘durham_region’;
else if (siteName === ‘guelphmercury.com’)
domain = ‘guelph_mercury’;
else if (siteName === ‘insidehalton.com’)
domain = ‘inside_halton’;
else if (siteName === ‘insideottawavalley.com’)
domain = ‘inside_ottawa_valley’;
else if (siteName === ‘mississauga.com’)
domain = ‘mississauga’;
else if (siteName === ‘muskokaregion.com’)
domain = ‘muskoka_region’;
else if (siteName === ‘newhamburgindependent.ca’)
domain = ‘new_hamburg_independent’;
else if (siteName === ‘niagarathisweek.com’)
domain = ‘niagara_this_week’;
else if (siteName === ‘northbaynipissing.com’)
domain = ‘north_bay_nipissing’;
else if (siteName === ‘northumberlandnews.com’)
domain = ‘northumberland_news’;
else if (siteName === ‘orangeville.com’)
domain = ‘orangeville’;
else if (siteName === ‘ourwindsor.ca’)
domain = ‘our_windsor’;
else if (siteName === ‘parrysound.com’)
domain = ‘parrysound’;
else if (siteName === ‘simcoe.com’)
domain = ‘simcoe’;
else if (siteName === ‘theifp.ca’)
domain = ‘the_ifp’;
else if (siteName === ‘waterloochronicle.ca’)
domain = ‘waterloo_chronicle’;
else if (siteName === ‘yorkregion.com’)
domain = ‘york_region’;
let sectionTag = ”;
try
if (domain === ‘thestar.com’ && path.indexOf(‘wires/’) = 0)
sectionTag = ‘/business’;
else if (path.indexOf(‘/autos’) >= 0)
sectionTag = ‘/autos’;
else if (path.indexOf(‘/entertainment’) >= 0)
sectionTag = ‘/entertainment’;
else if (path.indexOf(‘/life’) >= 0)
sectionTag = ‘/life’;
else if (path.indexOf(‘/news’) >= 0)
sectionTag = ‘/news’;
else if (path.indexOf(‘/politics’) >= 0)
sectionTag = ‘/politics’;
else if (path.indexOf(‘/sports’) >= 0)
sectionTag = ‘/sports’;
else if (path.indexOf(‘/opinion’) >= 0)
sectionTag = ‘/opinion’;
} catch (ex)
const descriptionUrl = ‘window.location.href’;
const vid = ‘mediainfo.reference_id’;
const cmsId = ‘2665777’;
let url = `https://pubads.g.doubleclick.net/gampad/ads?iu=/58580620/$domain/video/oovvuu$sectionTag&description_url=$descriptionUrl&vid=$vid&cmsid=$cmsId&tfcd=0&npa=0&sz=640×480&ad_rule=0&gdfp_req=1&output=vast&unviewed_position_start=1&env=vp&impl=s&correlator=`;
url = url.split(‘ ‘).join(”);
window.oovvuuReplacementAdServerURL = url;
Canada has seen a concerning rise in measles cases in the first months of 2024.
By the third week of March, the country had already recorded more than three times the number of cases as all of last year. Canada had just 12 cases of measles in 2023, up from three in 2022.
function buildUserSwitchAccountsForm()
var form = document.getElementById(‘user-local-logout-form-switch-accounts’);
if (form) return;
// build form with javascript since having a form element here breaks the payment modal.
var switchForm = document.createElement(‘form’);
switchForm.setAttribute(‘id’,’user-local-logout-form-switch-accounts’);
switchForm.setAttribute(‘method’,’post’);
switchForm.setAttribute(‘action’,’https://www.thestar.com/tncms/auth/logout/?return=https://www.thestar.com/users/login/?referer_url=https%3A%2F%2Fwww.thestar.com%2Fnews%2Fcanada%2Ftoronto-reports-2-more-measles-cases-use-our-tool-to-check-the-spread-in-canada%2Farticle_20aa7df4-e88f-11ee-8fad-8f8368d7ff53.html’);
switchForm.setAttribute(‘style’,’display:none;’);
var refUrl = document.createElement(‘input’); //input element, text
refUrl.setAttribute(‘type’,’hidden’);
refUrl.setAttribute(‘name’,’referer_url’);
refUrl.setAttribute(‘value’,’https://www.thestar.com/news/canada/toronto-reports-2-more-measles-cases-use-our-tool-to-check-the-spread-in-canada/article_20aa7df4-e88f-11ee-8fad-8f8368d7ff53.html’);
var submit = document.createElement(‘input’);
submit.setAttribute(‘type’,’submit’);
submit.setAttribute(‘name’,’logout’);
submit.setAttribute(‘value’,’Logout’);
switchForm.appendChild(refUrl);
switchForm.appendChild(submit);
document.getElementsByTagName(‘body’)[0].appendChild(switchForm);
function handleUserSwitchAccounts()
window.sessionStorage.removeItem(‘bd-viafoura-oidc’); // clear viafoura JWT token
// logout user before sending them to login page via return url
document.getElementById(‘user-local-logout-form-switch-accounts’).submit();
return false;
buildUserSwitchAccountsForm();
#ont-map-iframepadding:0;width:100%;border:0;overflow:hidden;
#ontario-cases-iframepadding:0;width:100%;border:0;overflow:hidden;
#province-table-iframepadding:0;width:100%;border:0;overflow:hidden;
console.log(‘=====> bRemoveLastParagraph: ‘,0);
Health
Cancer Awareness Month – Métis Nation of Alberta


Cancer Awareness Month
Posted on: Apr 18, 2024
April is Cancer Awareness Month
As we recognize Cancer Awareness Month, we stand together to raise awareness, support those affected, advocate for prevention, early detection, and continued research towards a cure. Cancer is the leading cause of death for Métis women and the second leading cause of death for Métis men. The Otipemisiwak Métis Government of the Métis Nation Within Alberta is working hard to ensure that available supports for Métis Citizens battling cancer are culturally appropriate, comprehensive, and accessible by Métis Albertans at all stages of their cancer journey.
Receiving a cancer diagnosis, whether for yourself or a loved one, can feel overwhelming, leaving you unsure of where to turn for support. In June, our government will be launching the Cancer Supports and Navigation Program which will further support Métis Albertans and their families experiencing cancer by connecting them to OMG-specific cancer resources, external resources, and providing navigation support through the health care system. This program will also include Métis-specific peer support groups for those affected by cancer.
With funding from the Canadian Partnership Against Cancer (CPAC) we have also developed the Métis Cancer Care Course to ensure that Métis Albertans have access to culturally safe and appropriate cancer services. This course is available to cancer care professionals across the country and provides an overview of who Métis people are, our culture, our approaches to health and wellbeing, our experiences with cancer care, and our cancer journey.
Together, we can make a difference in the fight against cancer and ensure equitable access to culturally safe and appropriate care for all Métis Albertans. Please click on the links below to learn more about the supports available for Métis Albertans, including our Compassionate Care: Cancer Transportation program.
I wish you all good health and happiness!
Bobbi Paul-Alook
Secretary of Health & Seniors
Health
Type 2 diabetes is not one-size-fits-all: Subtypes affect complications and treatment options – The Conversation


You may have heard of Ozempic, the “miracle drug” for weight loss, but did you know that it was actually designed as a new treatment to manage diabetes? In Canada, diabetes affects approximately 10 per cent of the general population. Of those cases, 90 per cent have Type 2 diabetes.
This metabolic disorder is characterized by persistent high blood sugar levels, which can be accompanied by secondary health challenges, including a higher risk of stroke and kidney disease.
Locks and keys
In Type 2 diabetes, the body struggles to maintain blood sugar levels in an acceptable range. Every cell in the body needs sugar as an energy source, but too much sugar can be toxic to cells. This equilibrium needs to be tightly controlled and is regulated by a lock and key system.
In the body’s attempt to manage blood sugar levels and ensure that cells receive the right amount of energy, the pancreatic hormone, insulin, functions like a key. Cells cover themselves with locks that respond perfectly to insulin keys to facilitate the entry of sugar into cells.
Unfortunately, this lock and key system doesn’t always perform as expected. The body can encounter difficulties producing an adequate number of insulin keys, and/or the locks can become stubborn and unresponsive to insulin.
All forms of diabetes share the challenge of high blood sugar levels; however, diabetes is not a singular condition; it exists as a spectrum. Although diabetes is broadly categorized into two main types, Type 1 and Type 2, each presents a diversity of subtypes, especially Type 2 diabetes.
These subtypes carry their own characteristics and risks, and do not respond uniformly to the same treatments.
To better serve people living with Type 2 diabetes, and to move away from a “one size fits all” approach, it is beneficial to understand which subtype of Type 2 diabetes a person lives with. When someone needs a blood transfusion, the medical team needs to know the patient’s blood type. It should be the same for diabetes so a tailored and effective game plan can be implemented.
This article explores four unique subtypes of Type 2 diabetes, shedding light on their causes, complications and some of their specific treatment avenues.
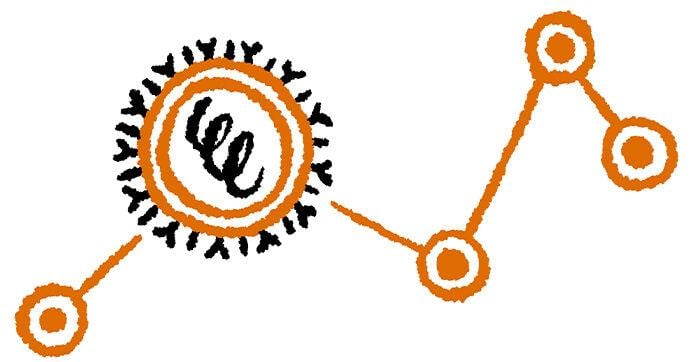
Severe insulin-deficient diabetes: We’re missing keys!
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Insulin is produced by beta cells, which are found in the pancreas. In the severe insulin-deficient diabetes (SIDD) subtype, the key factories — the beta cells — are on strike. Ultimately, there are fewer keys in the body to unlock the cells and allow entry of sugar from the blood.
SIDD primarily affects younger, leaner individuals, and unfortunately, increases the risk of eye disease and blindness, among other complications. Why the beta cells go on strike remains largely unknown, but since there is an insulin deficiency, treatment often involves insulin injections.
Severe insulin-resistant diabetes: But it’s always locked!
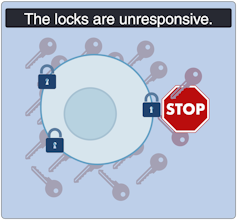
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
In the severe insulin-resistant diabetes (SIRD) subtype, the locks are overstimulated and start ignoring the keys. As a result, the beta cells produce even more keys to compensate. This can be measured as high levels of insulin in the blood, also known as hyperinsulinemia.
This resistance to insulin is particularly prominent in individuals with higher body weight. Patients with SIRD have an increased risk of complications such as fatty liver disease. There are many treatment avenues for these patients but no consensus about the optimal approach; patients often require high doses of insulin.
Mild obesity-related diabetes: The locks are sticky!
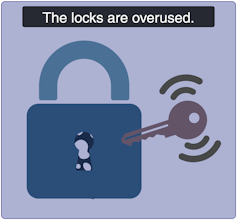
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Mild obesity-related (MOD) diabetes represents a nuanced aspect of Type 2 diabetes, often observed in individuals with higher body weight. Unlike more severe subtypes, MOD is characterized by a more measured response to insulin. The locks are “sticky,” so it is challenging for the key to click in place and open the lock. While MOD is connected to body weight, the comparatively less severe nature of MOD distinguishes it from other diabetes subtypes.
To minimize complications, treatment should include maintaining a healthy diet, managing body weight, and incorporating as much aerobic exercise as possible. This is where drugs like Ozempic can be prescribed to control the evolution of the disease, in part by managing body weight.
Mild age-related diabetes: I’m tired of controlling blood sugar!
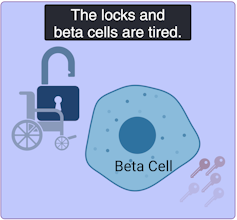
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Mild age-related diabetes (MARD) happens more often in older people and typically starts later in life. With time, the key factory is not as productive, and the locks become stubborn. People with MARD find it tricky to manage their blood sugar, but it usually doesn’t lead to severe complications.
Among the different subtypes of diabetes, MARD is the most common.
Unique locks, varied keys
While efforts have been made to classify diabetes subtypes, new subtypes are still being identified, making proper clinical assessment and treatment plans challenging.
In Canada, unique cases of Type 2 diabetes were identified in Indigenous children from Northern Manitoba and Northwestern Ontario by Dr. Heather Dean and colleagues in the 1980s and 90s. Despite initial skepticism from the scientific community, which typically associated Type 2 diabetes with adults rather than children, clinical teams persisted in identifying this as a distinct subtype of Type 2 diabetes, called childhood-onset Type 2 diabetes.
Read more:
Indigenous community research partnerships can help address health inequities
Childhood-onset Type 2 diabetes is on the rise across Canada, but disproportionately affects Indigenous youth. It is undoubtedly linked to the intergenerational trauma associated with colonization in these communities. While many factors are likely involved, recent studies have discovered that exposure of a fetus to Type 2 diabetes during pregnancy increases the risk that the baby will develop diabetes later in life.
Acknowledging this distinct subtype of Type 2 diabetes in First Nations communities has led to the implementation of a community-based health action plan aimed at addressing the unique challenges faced by Indigenous Peoples. It is hoped that partnered research between communities and researchers will continue to help us understand childhood-onset Type 2 diabetes and how to effectively prevent and treat it.
A mosaic of conditions

(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Type 2 diabetes is not uniform; it’s a mosaic of conditions, each with its own characteristics. Since diabetes presents so uniquely in every patient, even categorizing into subtypes does not guarantee how the disease will evolve. However, understanding these subtypes is a good starting point to help doctors create personalized plans for people living with the condition.
While Indigenous communities, lower-income households and individuals living with obesity already face a higher risk of developing Type 2 diabetes than the general population, tailored solutions may offer hope for better management. This emphasizes the urgent need for more precise assessments of diabetes subtypes to help customize therapeutic strategies and management strategies. This will improve care for all patients, including those from vulnerable and understudied populations.
-
Media5 hours ago
DJT Stock Rises. Trump Media CEO Alleges Potential Market Manipulation. – Barron's
-
Business23 hours ago
BC short-term rental rules take effect May 1 – CityNews Vancouver
-
Media7 hours ago
Trump Media alerts Nasdaq to potential market manipulation from 'naked' short selling of DJT stock – CNBC
-
Art22 hours ago
Collection of First Nations art stolen from Gordon Head home – Times Colonist
-



 Investment23 hours ago
Investment23 hours agoBenjamin Bergen: Why would anyone invest in Canada now? – National Post
-
Media19 hours ago
DJT Stock Jumps. The Truth Social Owner Is Showing Stockholders How to Block Short Sellers. – Barron's
-



 Health19 hours ago
Health19 hours agoType 2 diabetes is not one-size-fits-all: Subtypes affect complications and treatment options – The Conversation
-
Art22 hours ago
Crafting the Painterly Art Style in Eternal Strands – IGN First – IGN




