Health
What you need to know about COVID-19 in B.C. for Feb. 18 – CBC.ca

THE LATEST:
- Thursday’s COVID-19 update is expected in a written statement at around 3 p.m. PT
- As of Wednesday, there are 232 people in hospital with the disease, including 63 in intensive care.
- There are 4,150 active cases of the novel coronavirus across the province.
- A total of 1,317 people have died of COVID-19 in B.C. out of 74,710 confirmed cases.
- To date, 176,015 doses of vaccine have been administered, including 26,030 second doses.
Health officials are calling on British Columbians to treat each other with compassion while also encouraging those around them to follow public health orders and help get the second wave of COVID-19 under control.
In their daily update on Wednesday, Provincial Health Officer Dr. Bonnie Henry and Health Minister Adrian Dix said supporting others is crucial to protecting everyone’s physical and mental health.
“The COVID-19 pandemic has put a strain on all of us, but with kindness, compassion and care for those around us, we will see it through,” they said in a written statement.
“Most people in B.C. are doing the right thing, and we encourage everyone, in turn, to support friends and family to also continue to take precautions in their daily lives.”
As of Wednesday, there are 232 people in B.C. hospitals with COVID-19, including 63 in intensive care, out of 4,150 active cases across the province.
To date, 1,317 people have died as a result of infection with the novel coronavirus in B.C., out of 74,710 confirmed cases.
So far, 176,015 doses of COVID-19 vaccine have been administered, including 26,030 second doses.
On Wednesday, Dix said the province is expecting to receive 50,000 doses of the Pfizer vaccine this week, after it was delayed by a couple of days due to cold weather.
“We’ve delivered all of the immunizations really that we can in B.C. given the supply that’s been provided by the federal government,” said Dix, adding: “Hope is on the horizon.”
READ MORE:
What’s happening elsewhere in Canada
As of 6:30 p.m. PT Wednesday, Canada had reported 834,182 cases of COVID-19, with 32,986 cases considered active.
A total of 21,435 people have died.
What are the symptoms of COVID-19?
Common symptoms include:
- Fever.
- Cough.
- Tiredness.
- Shortness of breath.
- Loss of taste or smell.
- Headache.
But more serious symptoms can develop, including difficulty breathing and pneumonia.
What should I do if I feel sick?
Use the B.C. Centre for Disease Control’s COVID-19 self-assessment tool. Testing is recommended for anyone with symptoms of cold or flu, even if they’re mild. People with severe difficulty breathing, severe chest pain, difficulty waking up or other extreme symptoms should call 911.
What can I do to protect myself?
- Wash your hands frequently and thoroughly. Keep them clean.
- Keep at least two metres away from people outside your bubble. Keep your distance from people who are sick.
- Avoid touching your eyes, nose and mouth.
- Wear a mask in indoor public spaces.
- Be aware of evolving travel advisories to different regions.
More detailed information on the outbreak is available on the federal government’s website.
Health
Toronto reports 2 more measles cases. Use our tool to check the spread in Canada – Toronto Star

/* OOVVUU Targeting */
const path = ‘/news/canada’;
const siteName = ‘thestar.com’;
let domain = ‘thestar.com’;
if (siteName === ‘thestar.com’)
domain = ‘thestar.com’;
else if (siteName === ‘niagarafallsreview.ca’)
domain = ‘niagara_falls_review’;
else if (siteName === ‘stcatharinesstandard.ca’)
domain = ‘st_catharines_standard’;
else if (siteName === ‘thepeterboroughexaminer.com’)
domain = ‘the_peterborough_examiner’;
else if (siteName === ‘therecord.com’)
domain = ‘the_record’;
else if (siteName === ‘thespec.com’)
domain = ‘the_spec’;
else if (siteName === ‘wellandtribune.ca’)
domain = ‘welland_tribune’;
else if (siteName === ‘bramptonguardian.com’)
domain = ‘brampton_guardian’;
else if (siteName === ‘caledonenterprise.com’)
domain = ‘caledon_enterprise’;
else if (siteName === ‘cambridgetimes.ca’)
domain = ‘cambridge_times’;
else if (siteName === ‘durhamregion.com’)
domain = ‘durham_region’;
else if (siteName === ‘guelphmercury.com’)
domain = ‘guelph_mercury’;
else if (siteName === ‘insidehalton.com’)
domain = ‘inside_halton’;
else if (siteName === ‘insideottawavalley.com’)
domain = ‘inside_ottawa_valley’;
else if (siteName === ‘mississauga.com’)
domain = ‘mississauga’;
else if (siteName === ‘muskokaregion.com’)
domain = ‘muskoka_region’;
else if (siteName === ‘newhamburgindependent.ca’)
domain = ‘new_hamburg_independent’;
else if (siteName === ‘niagarathisweek.com’)
domain = ‘niagara_this_week’;
else if (siteName === ‘northbaynipissing.com’)
domain = ‘north_bay_nipissing’;
else if (siteName === ‘northumberlandnews.com’)
domain = ‘northumberland_news’;
else if (siteName === ‘orangeville.com’)
domain = ‘orangeville’;
else if (siteName === ‘ourwindsor.ca’)
domain = ‘our_windsor’;
else if (siteName === ‘parrysound.com’)
domain = ‘parrysound’;
else if (siteName === ‘simcoe.com’)
domain = ‘simcoe’;
else if (siteName === ‘theifp.ca’)
domain = ‘the_ifp’;
else if (siteName === ‘waterloochronicle.ca’)
domain = ‘waterloo_chronicle’;
else if (siteName === ‘yorkregion.com’)
domain = ‘york_region’;
let sectionTag = ”;
try
if (domain === ‘thestar.com’ && path.indexOf(‘wires/’) = 0)
sectionTag = ‘/business’;
else if (path.indexOf(‘/autos’) >= 0)
sectionTag = ‘/autos’;
else if (path.indexOf(‘/entertainment’) >= 0)
sectionTag = ‘/entertainment’;
else if (path.indexOf(‘/life’) >= 0)
sectionTag = ‘/life’;
else if (path.indexOf(‘/news’) >= 0)
sectionTag = ‘/news’;
else if (path.indexOf(‘/politics’) >= 0)
sectionTag = ‘/politics’;
else if (path.indexOf(‘/sports’) >= 0)
sectionTag = ‘/sports’;
else if (path.indexOf(‘/opinion’) >= 0)
sectionTag = ‘/opinion’;
} catch (ex)
const descriptionUrl = ‘window.location.href’;
const vid = ‘mediainfo.reference_id’;
const cmsId = ‘2665777’;
let url = `https://pubads.g.doubleclick.net/gampad/ads?iu=/58580620/$domain/video/oovvuu$sectionTag&description_url=$descriptionUrl&vid=$vid&cmsid=$cmsId&tfcd=0&npa=0&sz=640×480&ad_rule=0&gdfp_req=1&output=vast&unviewed_position_start=1&env=vp&impl=s&correlator=`;
url = url.split(‘ ‘).join(”);
window.oovvuuReplacementAdServerURL = url;
Canada has seen a concerning rise in measles cases in the first months of 2024.
By the third week of March, the country had already recorded more than three times the number of cases as all of last year. Canada had just 12 cases of measles in 2023, up from three in 2022.
function buildUserSwitchAccountsForm()
var form = document.getElementById(‘user-local-logout-form-switch-accounts’);
if (form) return;
// build form with javascript since having a form element here breaks the payment modal.
var switchForm = document.createElement(‘form’);
switchForm.setAttribute(‘id’,’user-local-logout-form-switch-accounts’);
switchForm.setAttribute(‘method’,’post’);
switchForm.setAttribute(‘action’,’https://www.thestar.com/tncms/auth/logout/?return=https://www.thestar.com/users/login/?referer_url=https%3A%2F%2Fwww.thestar.com%2Fnews%2Fcanada%2Ftoronto-reports-2-more-measles-cases-use-our-tool-to-check-the-spread-in-canada%2Farticle_20aa7df4-e88f-11ee-8fad-8f8368d7ff53.html’);
switchForm.setAttribute(‘style’,’display:none;’);
var refUrl = document.createElement(‘input’); //input element, text
refUrl.setAttribute(‘type’,’hidden’);
refUrl.setAttribute(‘name’,’referer_url’);
refUrl.setAttribute(‘value’,’https://www.thestar.com/news/canada/toronto-reports-2-more-measles-cases-use-our-tool-to-check-the-spread-in-canada/article_20aa7df4-e88f-11ee-8fad-8f8368d7ff53.html’);
var submit = document.createElement(‘input’);
submit.setAttribute(‘type’,’submit’);
submit.setAttribute(‘name’,’logout’);
submit.setAttribute(‘value’,’Logout’);
switchForm.appendChild(refUrl);
switchForm.appendChild(submit);
document.getElementsByTagName(‘body’)[0].appendChild(switchForm);
function handleUserSwitchAccounts()
window.sessionStorage.removeItem(‘bd-viafoura-oidc’); // clear viafoura JWT token
// logout user before sending them to login page via return url
document.getElementById(‘user-local-logout-form-switch-accounts’).submit();
return false;
buildUserSwitchAccountsForm();
#ont-map-iframepadding:0;width:100%;border:0;overflow:hidden;
#ontario-cases-iframepadding:0;width:100%;border:0;overflow:hidden;
#province-table-iframepadding:0;width:100%;border:0;overflow:hidden;
console.log(‘=====> bRemoveLastParagraph: ‘,0);
Health
Cancer Awareness Month – Métis Nation of Alberta


Cancer Awareness Month
Posted on: Apr 18, 2024
April is Cancer Awareness Month
As we recognize Cancer Awareness Month, we stand together to raise awareness, support those affected, advocate for prevention, early detection, and continued research towards a cure. Cancer is the leading cause of death for Métis women and the second leading cause of death for Métis men. The Otipemisiwak Métis Government of the Métis Nation Within Alberta is working hard to ensure that available supports for Métis Citizens battling cancer are culturally appropriate, comprehensive, and accessible by Métis Albertans at all stages of their cancer journey.
Receiving a cancer diagnosis, whether for yourself or a loved one, can feel overwhelming, leaving you unsure of where to turn for support. In June, our government will be launching the Cancer Supports and Navigation Program which will further support Métis Albertans and their families experiencing cancer by connecting them to OMG-specific cancer resources, external resources, and providing navigation support through the health care system. This program will also include Métis-specific peer support groups for those affected by cancer.
With funding from the Canadian Partnership Against Cancer (CPAC) we have also developed the Métis Cancer Care Course to ensure that Métis Albertans have access to culturally safe and appropriate cancer services. This course is available to cancer care professionals across the country and provides an overview of who Métis people are, our culture, our approaches to health and wellbeing, our experiences with cancer care, and our cancer journey.
Together, we can make a difference in the fight against cancer and ensure equitable access to culturally safe and appropriate care for all Métis Albertans. Please click on the links below to learn more about the supports available for Métis Albertans, including our Compassionate Care: Cancer Transportation program.
I wish you all good health and happiness!
Bobbi Paul-Alook
Secretary of Health & Seniors
Health
Type 2 diabetes is not one-size-fits-all: Subtypes affect complications and treatment options – The Conversation


You may have heard of Ozempic, the “miracle drug” for weight loss, but did you know that it was actually designed as a new treatment to manage diabetes? In Canada, diabetes affects approximately 10 per cent of the general population. Of those cases, 90 per cent have Type 2 diabetes.
This metabolic disorder is characterized by persistent high blood sugar levels, which can be accompanied by secondary health challenges, including a higher risk of stroke and kidney disease.
Locks and keys
In Type 2 diabetes, the body struggles to maintain blood sugar levels in an acceptable range. Every cell in the body needs sugar as an energy source, but too much sugar can be toxic to cells. This equilibrium needs to be tightly controlled and is regulated by a lock and key system.
In the body’s attempt to manage blood sugar levels and ensure that cells receive the right amount of energy, the pancreatic hormone, insulin, functions like a key. Cells cover themselves with locks that respond perfectly to insulin keys to facilitate the entry of sugar into cells.
Unfortunately, this lock and key system doesn’t always perform as expected. The body can encounter difficulties producing an adequate number of insulin keys, and/or the locks can become stubborn and unresponsive to insulin.
All forms of diabetes share the challenge of high blood sugar levels; however, diabetes is not a singular condition; it exists as a spectrum. Although diabetes is broadly categorized into two main types, Type 1 and Type 2, each presents a diversity of subtypes, especially Type 2 diabetes.
These subtypes carry their own characteristics and risks, and do not respond uniformly to the same treatments.
To better serve people living with Type 2 diabetes, and to move away from a “one size fits all” approach, it is beneficial to understand which subtype of Type 2 diabetes a person lives with. When someone needs a blood transfusion, the medical team needs to know the patient’s blood type. It should be the same for diabetes so a tailored and effective game plan can be implemented.
This article explores four unique subtypes of Type 2 diabetes, shedding light on their causes, complications and some of their specific treatment avenues.
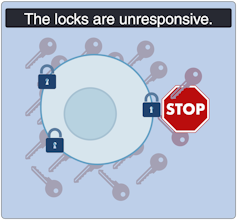
Severe insulin-deficient diabetes: We’re missing keys!
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Insulin is produced by beta cells, which are found in the pancreas. In the severe insulin-deficient diabetes (SIDD) subtype, the key factories — the beta cells — are on strike. Ultimately, there are fewer keys in the body to unlock the cells and allow entry of sugar from the blood.
SIDD primarily affects younger, leaner individuals, and unfortunately, increases the risk of eye disease and blindness, among other complications. Why the beta cells go on strike remains largely unknown, but since there is an insulin deficiency, treatment often involves insulin injections.
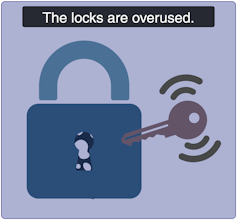
Severe insulin-resistant diabetes: But it’s always locked!
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
In the severe insulin-resistant diabetes (SIRD) subtype, the locks are overstimulated and start ignoring the keys. As a result, the beta cells produce even more keys to compensate. This can be measured as high levels of insulin in the blood, also known as hyperinsulinemia.
This resistance to insulin is particularly prominent in individuals with higher body weight. Patients with SIRD have an increased risk of complications such as fatty liver disease. There are many treatment avenues for these patients but no consensus about the optimal approach; patients often require high doses of insulin.
Mild obesity-related diabetes: The locks are sticky!
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Mild obesity-related (MOD) diabetes represents a nuanced aspect of Type 2 diabetes, often observed in individuals with higher body weight. Unlike more severe subtypes, MOD is characterized by a more measured response to insulin. The locks are “sticky,” so it is challenging for the key to click in place and open the lock. While MOD is connected to body weight, the comparatively less severe nature of MOD distinguishes it from other diabetes subtypes.
To minimize complications, treatment should include maintaining a healthy diet, managing body weight, and incorporating as much aerobic exercise as possible. This is where drugs like Ozempic can be prescribed to control the evolution of the disease, in part by managing body weight.
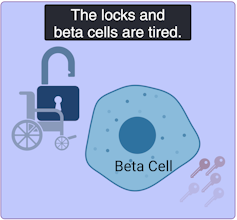
Mild age-related diabetes: I’m tired of controlling blood sugar!
(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Mild age-related diabetes (MARD) happens more often in older people and typically starts later in life. With time, the key factory is not as productive, and the locks become stubborn. People with MARD find it tricky to manage their blood sugar, but it usually doesn’t lead to severe complications.
Among the different subtypes of diabetes, MARD is the most common.
Unique locks, varied keys
While efforts have been made to classify diabetes subtypes, new subtypes are still being identified, making proper clinical assessment and treatment plans challenging.
In Canada, unique cases of Type 2 diabetes were identified in Indigenous children from Northern Manitoba and Northwestern Ontario by Dr. Heather Dean and colleagues in the 1980s and 90s. Despite initial skepticism from the scientific community, which typically associated Type 2 diabetes with adults rather than children, clinical teams persisted in identifying this as a distinct subtype of Type 2 diabetes, called childhood-onset Type 2 diabetes.
Read more:
Indigenous community research partnerships can help address health inequities
Childhood-onset Type 2 diabetes is on the rise across Canada, but disproportionately affects Indigenous youth. It is undoubtedly linked to the intergenerational trauma associated with colonization in these communities. While many factors are likely involved, recent studies have discovered that exposure of a fetus to Type 2 diabetes during pregnancy increases the risk that the baby will develop diabetes later in life.
Acknowledging this distinct subtype of Type 2 diabetes in First Nations communities has led to the implementation of a community-based health action plan aimed at addressing the unique challenges faced by Indigenous Peoples. It is hoped that partnered research between communities and researchers will continue to help us understand childhood-onset Type 2 diabetes and how to effectively prevent and treat it.
A mosaic of conditions

(Lili Grieco-St-Pierre, Jennifer Bruin/Created with BioRender.com)
Type 2 diabetes is not uniform; it’s a mosaic of conditions, each with its own characteristics. Since diabetes presents so uniquely in every patient, even categorizing into subtypes does not guarantee how the disease will evolve. However, understanding these subtypes is a good starting point to help doctors create personalized plans for people living with the condition.
While Indigenous communities, lower-income households and individuals living with obesity already face a higher risk of developing Type 2 diabetes than the general population, tailored solutions may offer hope for better management. This emphasizes the urgent need for more precise assessments of diabetes subtypes to help customize therapeutic strategies and management strategies. This will improve care for all patients, including those from vulnerable and understudied populations.
-



 Investment24 hours ago
Investment24 hours agoUK Mulls New Curbs on Outbound Investment Over Security Risks – BNN Bloomberg
-



 Sports22 hours ago
Sports22 hours agoAuston Matthews denied 70th goal as depleted Leafs lose last regular-season game – Toronto Sun
-
Media3 hours ago
DJT Stock Rises. Trump Media CEO Alleges Potential Market Manipulation. – Barron's
-
Business21 hours ago
BC short-term rental rules take effect May 1 – CityNews Vancouver
-
Media5 hours ago
Trump Media alerts Nasdaq to potential market manipulation from 'naked' short selling of DJT stock – CNBC
-
Art20 hours ago
Collection of First Nations art stolen from Gordon Head home – Times Colonist
-



 Investment21 hours ago
Investment21 hours agoBenjamin Bergen: Why would anyone invest in Canada now? – National Post
-



 Tech23 hours ago
Tech23 hours agoSave $700 Off This 4K Projector at Amazon While You Still Can – CNET






