-
Tobón, G. J., Youinou, P. & Saraux, A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 35, 10–14 (2010).
Article
PubMed
Google Scholar
-
Dejaco, C., Duftner, C., Grubeck-Loebenstein, B. & Schirmer, M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology 117, 289–300 (2006).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
De Almeida, D. E. et al. Immune dysregulation by the rheumatoid arthritis shared epitope. J. Immunol. Baltim. Md 1950(185), 1927–1934 (2010).
Google Scholar
-
Yap, H.-Y. et al. Pathogenic role of immune cells in rheumatoid arthritis: Implications in clinical treatment and biomarker development. Cells 7, 161 (2018).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Afzali, B., Lombardi, G., Lechler, R. I. & Lord, G. M. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin. Exp. Immunol. 148, 32–46 (2007).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Martinez, G. J., Nurieva, R. I., Yang, X. O. & Dong, C. Regulation and function of proinflammatory TH17 cells. Ann. N. Y. Acad. Sci. 1143, 188–211 (2008).
Article
ADS
CAS
PubMed
PubMed Central
Google Scholar
-
Corthay, A. How do regulatory T cells work?. Scand. J. Immunol. 70, 326–336 (2009).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Capone, A. & Volpe, E. Transcriptional regulators of T helper 17 cell differentiation in health and autoimmune diseases. Front. Immunol. 11, 348 (2020).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Pereira, L. M. S., Gomes, S. T. M., Ishak, R. & Vallinoto, A. C. R. Regulatory T cell and forkhead box protein 3 as modulators of immune homeostasis. Front. Immunol. 8, 605 (2017).
Article
PubMed
PubMed Central
Google Scholar
-
Jiang, Q., Yang, G., Liu, Q., Wang, S. & Cui, D. Function and role of regulatory T cells in rheumatoid arthritis. Front. Immunol. 12, 626193 (2021).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Lee, G. R. The balance of Th17 versus Treg cells in autoimmunity. Int. J. Mol. Sci. 19, 730 (2018).
Article
PubMed
PubMed Central
Google Scholar
-
Weyand, C. M., Yang, Z. & Goronzy, J. J. T cell aging in rheumatoid arthritis. Curr. Opin. Rheumatol. 26, 93–100 (2014).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Chalan, P., van den Berg, A., Kroesen, B.-J., Brouwer, L. & Boots, A. Rheumatoid arthritis, immunosenescence and the hallmarks of aging. Curr. Aging Sci. 8, 131–146 (2015).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Goronzy, J. J. & Weyand, C. M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 19, 573–583 (2019).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Shao, L. et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206, 1435–1449 (2009).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Li, Y. et al. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity 45, 903–916 (2016).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Belk, J. A., Daniel, B. & Satpathy, A. T. Epigenetic regulation of T cell exhaustion. Nat. Immunol. 23, 848–860 (2022).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Gautam, S. & Dada, R. Molecular mechanisms underlying the effects of yoga. In Handbook of Research on Evidence-Based Perspectives on the Psychophysiology of Yoga and Its Applications. 103–123. https://www.igi-global.com/chapter/molecular-mechanisms-underlying-the-effects-of-yoga/www.igi-global.com/chapter/molecular-mechanisms-underlying-the-effects-of-yoga/261146. https://doi.org/10.4018/978-1-7998-3254-6.ch007 (2021).
-
Gautam, S., Tolahunase, M., Kumar, U. & Dada, R. Impact of yoga-based mind-body intervention on systemic inflammatory markers and co-morbid depression in active rheumatoid arthritis patients: A randomized controlled trial. Restor. Neurol. Neurosci. 37, 41–59 (2019).
CAS
PubMed
Google Scholar
-
Gautam, S., Kumar, M., Kumar, U. & Dada, R. Effect of an 8-week yoga-based lifestyle intervention on psycho-neuro-immune axis, disease activity, and perceived quality of life in rheumatoid arthritis patients: A randomized controlled trial. Front. Psychol. 11, 2259 (2020).
Article
PubMed
PubMed Central
Google Scholar
-
Gautam, S., Kumar, U. & Dada, R. Yoga and its impact on chronic inflammatory autoimmune arthritis. Front. Biosci. Elite Ed. 13, 77–116 (2021).
Article
PubMed
Google Scholar
-
Arora, S. & Bhattacharjee, J. Modulation of immune responses in stress by yoga. Int. J. Yoga 1, 45–55 (2008).
Article
PubMed
PubMed Central
Google Scholar
-
Falkenberg, R. I., Eising, C. & Peters, M. L. Yoga and immune system functioning: A systematic review of randomized controlled trials. J. Behav. Med. 41, 467–482 (2018).
Article
CAS
PubMed
Google Scholar
-
Gautam, S., Kumar, U., Kumar, M., Rana, D. & Dada, R. Yoga improves mitochondrial health and reduces severity of autoimmune inflammatory arthritis: A randomized controlled trial. Mitochondrion 58, 147–159 (2021).
Article
CAS
PubMed
Google Scholar
-
Gautam, S., Kumar, U., Mishra, R. & Dada, R. HLA-G 3’UTR polymorphisms & response to a yoga-based lifestyle intervention in rheumatoid arthritis: A randomized controlled trial. Indian J. Med. Res. 155, 253–263 (2022).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Kay, J. & Upchurch, K. S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatol. Oxf. Engl. 51(Suppl 6), vi5-9 (2012).
Article
Google Scholar
-
Evans, S. et al. A randomized controlled trial examining Iyengar yoga for young adults with rheumatoid arthritis: a study protocol. Trials 12, 19 (2011).
Article
PubMed
PubMed Central
Google Scholar
-
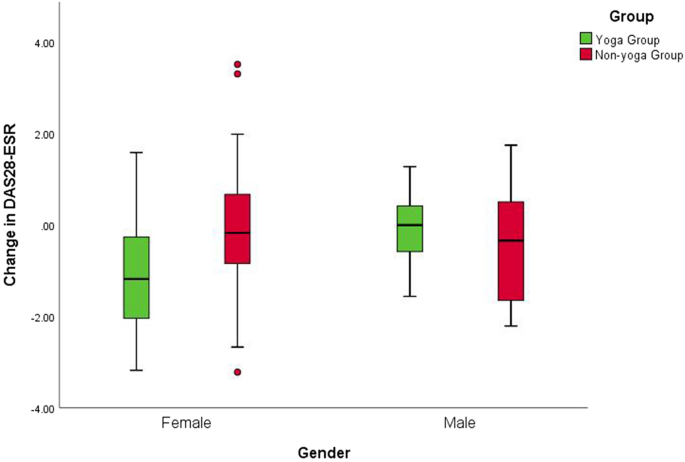
Nishimoto, N. & Takagi, N. Assessment of the validity of the 28-joint disease activity score using erythrocyte sedimentation rate (DAS28-ESR) as a disease activity index of rheumatoid arthritis in the efficacy evaluation of 24-week treatment with tocilizumab: subanalysis of the SATORI study. Mod. Rheumatol. 20, 539–547 (2010).
Article
PubMed
PubMed Central
Google Scholar
-
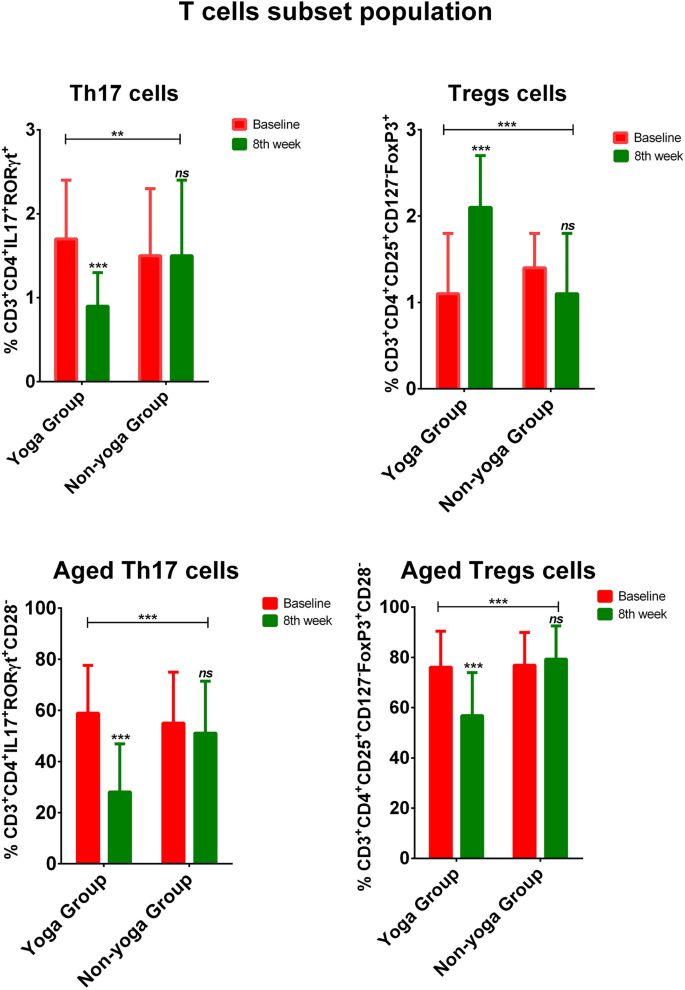
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods San Diego Calif. 25, 402–408 (2001).
Article
CAS
PubMed
Google Scholar
-
Yoshida, Y. & Tanaka, T. Interleukin 6 and rheumatoid arthritis. BioMed Res. Int. 2014, 698313 (2014).
Article
PubMed
PubMed Central
Google Scholar
-
Cronstein, B. N. Interleukin-6—A key mediator of systemic and local symptoms in rheumatoid arthritis. Bull. NYU Hosp. Jt. Dis. 65(Suppl 1), S11-15 (2007).
PubMed
Google Scholar
-
Guo, Q. et al. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15 (2018).
Article
ADS
PubMed
PubMed Central
Google Scholar
-
O’Brien, W. et al. Receptor activator of nuclear factor kappa-B (RANK) independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. Hoboken NJ 68, 2889–2900 (2016).
Article
Google Scholar
-
Robert, M. & Miossec, P. IL-17 in rheumatoid arthritis and precision medicine: From synovitis expression to circulating bioactive levels. Front. Med. 5, 364 (2019).
Article
Google Scholar
-
Tesmer, L. A., Lundy, S. K., Sarkar, S. & Fox, D. A. Th17 cells in human disease. Immunol. Rev. 223, 87–113 (2008).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Shabgah, A. G., Fattahi, E. & Shahneh, F. Z. Interleukin-17 in human inflammatory diseases. Postepy Dermatol. Alergol. 31, 256–261 (2014).
Article
PubMed
PubMed Central
Google Scholar
-
Wang, X. et al. Increased expression of CXCL2 in ACPA-positive rheumatoid arthritis and its role in osteoclastogenesis. Clin. Exp. Immunol. 203, 194–208 (2021).
Article
CAS
PubMed
Google Scholar
-
Jacobs, J. P. et al. Deficiency of CXCR2, but not other chemokine receptors, attenuates a murine model of autoantibody-mediated arthritis. Arthritis Rheum. 62, 1921–1932 (2010).
CAS
PubMed
PubMed Central
Google Scholar
-
Woodyard, C. Exploring the therapeutic effects of yoga and its ability to increase quality of life. Int. J. Yoga 4, 49–54 (2011).
Article
PubMed
PubMed Central
Google Scholar
-
Vijayaraghava, A., Doreswamy, V., Narasipur, O. S., Kunnavil, R. & Srinivasamurthy, N. Effect of yoga practice on levels of inflammatory markers after moderate and strenuous exercise. J. Clin. Diagn. Res. JCDR 9, CC08-12 (2015).
PubMed
Google Scholar
-
Taylor, A., Verhagen, J., Blaser, K., Akdis, M. & Akdis, C. A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: The role of T regulatory cells. Immunology 117, 433–442 (2006).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Article
CAS
PubMed
Google Scholar
-
Zhang, S. The role of transforming growth factor β in T helper 17 differentiation. Immunology 155, 24–35 (2018).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Aarts, J. et al. Local inhibition of TGF-β1 signaling improves Th17/Treg balance but not joint pathology during experimental arthritis. Sci. Rep. 12, 3182 (2022).
Article
ADS
CAS
PubMed
PubMed Central
Google Scholar
-
Garces de losFayos Alonso, I. et al. The role of activator protein-1 (AP-1) family members in CD30-positive lymphomas. Cancers 10, 93 (2018).
Article
Google Scholar
-
Johnston, I. M. et al. Regulation of a multigenic invasion programme by the transcription factor, AP-1: Re-expression of a down-regulated gene, TSC-36, inhibits invasion. Oncogene 19, 5348–5358 (2000).
Article
CAS
PubMed
Google Scholar
-
Oh, B. et al. The effects of Tai Chi and Qigong on immune responses: A systematic review and meta-analysis. Medicines 7, 39 (2020).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Wang, M.-Y. & An, L.-G. Effects of 12-Week’s Tai Chi Chuan Practice on the Immune Function of Female College Students Who Lack Physical Exercise. https://doaj.org/article/51b2fc58e04542e2b3224c586645a1b2 (2011).
-
Chen, Z. et al. Effect of aerobic exercise on Treg and Th17 of rats with ischemic cardiomyopathy. J Cardiovasc. Transl. Res. 11, 230–235 (2018).
Article
PubMed
Google Scholar
-
Perry, C. et al. Endurance exercise diverts the balance between Th17 cells and regulatory T cells. PLoS ONE 8, e74722 (2013).
Article
ADS
CAS
PubMed
PubMed Central
Google Scholar
-
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Article
PubMed
PubMed Central
Google Scholar
-
Beyersdorf, N., Kerkau, T. & Hünig, T. CD28 co-stimulation in T-cell homeostasis: A recent perspective. ImmunoTargets Ther. 4, 111–122 (2015).
PubMed
PubMed Central
Google Scholar
-
Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 90, 9–17 (2011).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Turner, B. M. Epigenetic responses to environmental change and their evolutionary implications. Philos. Trans. R. Soc. B Biol. Sci. 364, 3403–3418 (2009).
Article
CAS
Google Scholar
-
Nemtsova, M. V. et al. Epigenetic changes in the pathogenesis of rheumatoid arthritis. Front. Genet. 10, 570 (2019).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Ramos-Lopez, O., Milagro, F. I., Riezu-Boj, J. I. & Martinez, J. A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 70, 29–49 (2021).
Article
CAS
PubMed
Google Scholar
-
de Andres, M. C. et al. Assessment of global DNA methylation in peripheral blood cell subpopulations of early rheumatoid arthritis before and after methotrexate. Arthritis Res. Ther. 17, 233 (2015).
Article
PubMed
PubMed Central
Google Scholar
-
Jeffries, M. A. & Sawalha, A. H. Autoimmune disease in the epigenetic era: How has epigenetics changed our understanding of disease and how can we expect the field to evolve?. Expert Rev. Clin. Immunol. 11, 45–58 (2015).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Karouzakis, E., Gay, R. E., Michel, B. A., Gay, S. & Neidhart, M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 60, 3613–3622 (2009).
Article
CAS
PubMed
Google Scholar
-
Karouzakis, E. et al. Analysis of early changes in DNA methylation in synovial fibroblasts of RA patients before diagnosis. Sci. Rep. 8, 7370 (2018).
Article
ADS
PubMed
PubMed Central
Google Scholar
-
Kriaucionis, S. & Heintz, N. The nuclear DNA base, 5-hydroxymethylcytosine is present in brain and enriched in Purkinje neurons. Science 324, 929–930 (2009).
Article
ADS
CAS
PubMed
PubMed Central
Google Scholar
-
Handy, D. E., Castro, R. & Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 123, 2145–2156 (2011).
Article
PubMed
PubMed Central
Google Scholar
-
Seto, E. & Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 6, 18713 (2014).
Article
Google Scholar
-
Vojinovic, J. & Damjanov, N. HDAC inhibition in rheumatoid arthritis and juvenile idiopathic arthritis. Mol. Med. 17, 397–403 (2011).
Article
CAS
PubMed
PubMed Central
Google Scholar
-
Grabiec, A. M., Korchynskyi, O., Tak, P. P. & Reedquist, K. A. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann. Rheum. Dis. 71, 424–431 (2012).
Article
CAS
PubMed
Google Scholar
-
Hull, E. E., Montgomery, M. R. & Leyva, K. J. HDAC inhibitors as epigenetic regulators of the immune system: Impacts on cancer therapy and inflammatory diseases. BioMed Res. Int. 2016, 8797206 (2016).
Article
PubMed
PubMed Central
Google Scholar