CHICAGO, IL—The use of botulinum toxin type A in patients undergoing cardiac surgery does not seem to reduce the rate of postoperative atrial fibrillation (AF) overall, according to the phase II NOVA study. But the trial did show hints that it could provide benefit in certain subgroups.
Postoperative AF affects between one- and two-thirds of patients following cardiac surgery and can portend the same risk of stoke as AF in other settings.
“There is an unmet need for therapies that can effectively and safely reduce the occurrence of postoperative atrial fibrillation,” said Jonathan Piccini, MD (Duke Clinical Research Institute, Durham, NC), who presented the findings today at a late-breaking clinical trial session at the American Heart Association (AHA) 2022 Scientific Sessions. “Suppression of atrial fibrillation with botulinum toxin is likely mediated through both direct autonomic effects as well as reductions in inflammation,” he explained.
Commenting on the findings for TCTMD, Konstantinos C. Siontis, MD (Mayo Clinic, Rochester, MN), said NOVA is “a highly anticipated study that provides dose-specific safety and effectiveness data.” However, he said in an email, “due to sample size and event rates, it is still difficult to reach conclusions regarding clinically meaningful outcomes, such as episodes of AF lasting more than a few seconds or minutes.”
Phase II Results
For the study, Piccini and colleagues randomized 323 patients (mean age 67 years; 83% male) undergoing cardiac surgery to receive epicardial injections of botulinum toxin type A either in doses of 125 units (n = 106) or 250 units (n = 109) or placebo (n = 108). Roughly two-thirds of patients underwent CABG, one-quarter had valve repair/replacement, and the remaining 12% had both. All patients were in sinus rhythm for at least 48 hours prior to surgery and were willing to wear an ECG patch for 30 days postsurgery and for 7 days after each study visit.
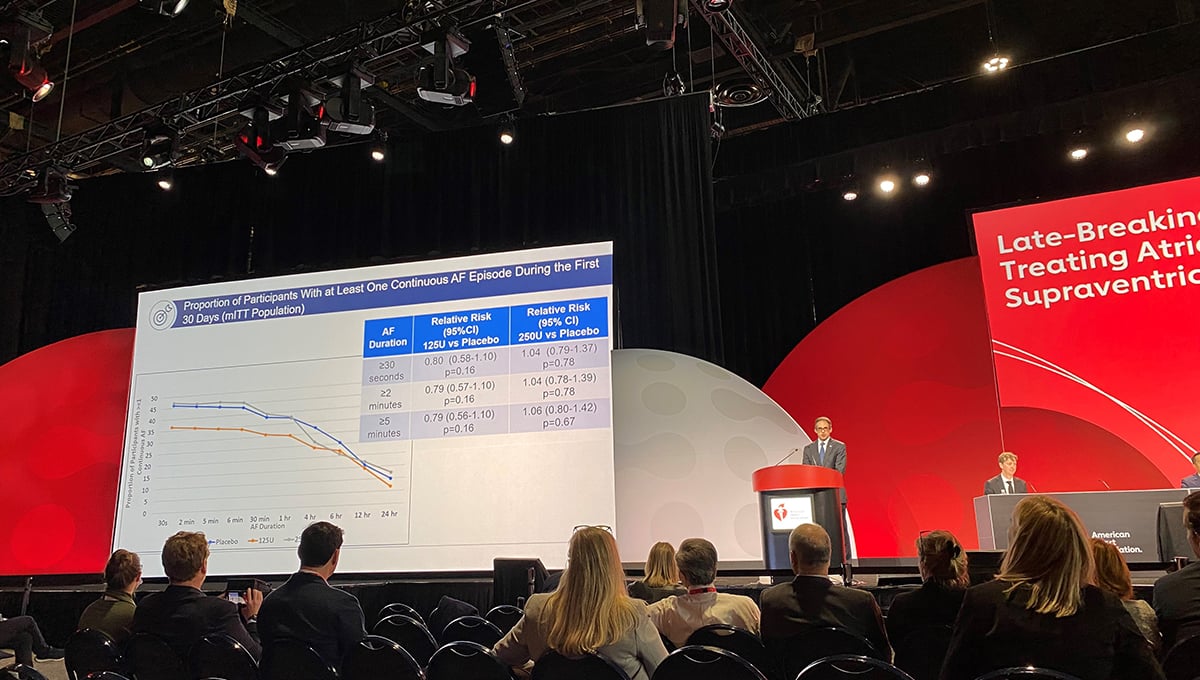
Within the first 30 days, there were no differences in the rate of AF lasting for at least 30 seconds (primary endpoint) for patients treated with either the 125-unit (RR 0.80; 95% CI 0.58-1.10) or 250-unit dose (RR 1.04; 95% CI 0.79-1.37) of botulinum toxin type A compared with placebo.
There was a trend observed for a benefit with the 125-unit dose compared with placebo in those undergoing isolated CABG (RR 0.71; 95% CI 0.44-1.15), and a significant benefit observed in the subgroup of patients aged at least 65 years old receiving the 125-unit dose with regards to AF episodes lasting at least 30 seconds (RR 0.64; 95% CI 0.43-0.94), 2 minutes (RR 0.63; 95% CI 0.42-0.94), and 5 minutes (RR 0.64; 95% CI 0.43-0.97).
The mean length of hospital stay was similar for all patient groups, ranging between 6.4 and 6.6 days. However, there were numeric reductions in all-cause rehospitalizations for those receiving 125 and 250 units of the study drug—with rates of 8.7% and 9.4%, respectively—compared with patients who got placebo (15.7%).
All patients in the CABG subgroup saw reductions in interleukin-6 regardless of study arm, and those receiving either dose of botulinum toxin type A saw reductions in high-sensitivity C-reactive protein compared with placebo.
Lastly, the rates of adverse events were similar for the three groups (ranging from 88.6% to 95.4%), as were rates of treatment-emergent adverse events (49.5% to 61.9%). The majority of events were supraventricular arrhythmias.
Piccini highlighted that because the study was a “phase II dose ranging exploratory clinical study, it was not powered to discern all clinically relevant differences in postoperative atrial fibrillation, nor was it powered to discern to certain differences in cardiovascular outcomes.” Also, he continued, “analyses of subgroups were limited due to sample size.”
More Mechanistic Insight Desired
Siontis called the dose-specific data “intriguing” and said he was somewhat surprised to see that the lower dose of the study drug had a greater potential effect on postoperative AF compared with the higher dose. “This is unexpected at first glance but might also suggest that too much cholinergic inhibition could be counterproductive,” he suggested.
Discussing the study following the presentation, Usha Tedrow, MD (Brigham and Women’s Hospital, Boston, MA), said that because not even a hint of benefit was observed for the 250-unit dose of botulinum toxin type A, that “raises some questions about the mechanism of the reductions in atrial fibrillation events.”
Also, she said, “reduction in inflammatory markers was seen for both doses and raises the question whether the AF reduction was maybe not mediated by inflammation specifically.”
Tedrow said that in the future she would like to see more information about patient heart-rate variability or other forms of autonomic assessment in order to better understand the mechanism of action of how the botulinum toxin affects AF occurrence.
For Siontis, a closer look into the decrease in all-cause hospitalizations would be a useful way to tease out whether the reduction was due to fewer arrhythmias or perhaps “other collateral benefits” botulinum toxin. Additionally, he continued, “it would be great to see larger, well powered clinical trials that can address major arrhythmia-related clinical endpoints, with emphasis on higher-risk patients, including those older than 60-65 years. Also [welcome are] more clinical trials testing noninvasive, extracardiac autonomic modulation to reduce postoperative AF after cardiac surgery.”