News
Grocery bills set to increase for Canadians: report – Global News
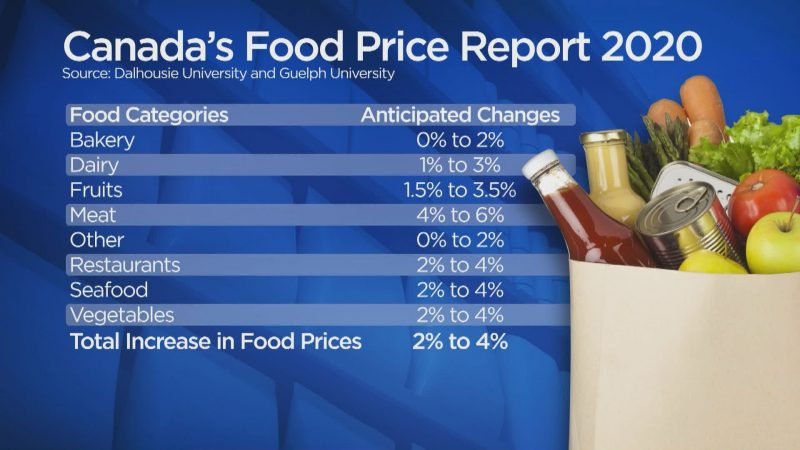
According to a new report from Dalhousie University and the University of Guelph, the average Canadian family is set to spend a lot more at the grocery store.
Each year, Canada’s Food Price Report predicts changes in the major food categories and outlines the factors, both expected and unforeseen, that influence the cost to the consumer.
Read more:
Despite the pandemic and a drought, many Peterborough producers reporting strong selling season
This year’s report forecasts that overall food prices in Canada will increase between two and four per cent, and that the average Canadian family will spend $12,667 on their annual grocery bill — an increase of $487 compared to the 2019 model.
Canada’s Food Price Report 2020 showing a prediction of changes in price for major food categories.
One of the events that has triggered this rise in price is the COVID-19 pandemic.
“The reason that we released the report this week is that we actually believe that COVID could make things worse,” said Sylvain Charlebois, director of the Agri-Food Analytics Lab at Dalhousie University, and the report’s lead author.
[ Sign up for our Health IQ newsletter for the latest coronavirus updates ]
“Right now the food inflation rate is at about two per cent, while the general inflation rate is at zero per cent.
“You can see that really over time — because of COVID and because of the economy — it will get more expensive at the grocery store, while for everything else, things are probably going to remain the same for a while.”

Canada’s Food Price Report 2020 outlining which provinces will be above, below or at the food price national average.
In addition to the coronavirus pandemic, the report also sees climate change as the “elephant in the room,” and how events such as droughts and forest fires, as well as a rise in ocean levels, all play a role in affecting our food systems.
“[Wildfires in California] are certainly a big factor for the west,” Charlebois said.
“Canada does buy about $3 billion worth of food wholesale every year from California, but a lot of it actually goes to the west — Alberta, B.C. — between October and January.
“We are expecting that phenomenon to impact food prices, and not just out west, but everywhere in Canada.”
Something else that Charlebois says was identified from creating this year’s report is that the food inflation index has outpaced the general inflation rate by about 16 points overall over the last 20 years.

During this time, one item that has continued to grow in price is baby food.
“The demand elasticity for baby food is very high. If you buy it, you need it, and you won’t necessarily look at the price,” Charlebois explained.
Conversely, the price of a jar of peanut butter has remained relatively the same price, or even cheaper, year-over-year since 2000, which Charlebois says is due to increased competition from other brands and private labels.
© 2020 Global News, a division of Corus Entertainment Inc.
News
Health Canada sperm donation rules changing for gay men – CTV News


Health Canada will change its longstanding policy restricting gay and bisexual men from donating to sperm banks in Canada, CTV News has learned.
The federal health agency has adopted a revised directive removing the ban on gay, bisexual and other men who have sex with men, effective May 8.
The policy change would remove the current donor screening criteria, allowing men who have sex with men to legally donate sperm for the first time in more than 30 years, as part of the anonymous donation process.
This update comes after CTV News first reported last year that a gay man was taking the federal government to court, challenging the constitutionality of the policy on the basis that it violates the right to equality in the Charter of Rights and Freedoms.
According to an email Health Canada sent stakeholders informing them of the upcoming amendments to the federal directive, “sperm donors will instead be asked gender-neutral, sexual behaviour-based donor screening questions,” more in-line with the 2022 change made by Canadian Blood Services to its donation policy.
However, instead of entirely eradicating restrictions for gay and bisexual men, lawyer Gregory Ko – whose client, Aziz M., brought the case – cautioned that Health Canada will continue to bar donations from those who have had new or multiple partners in the last three months, based on rules regarding anal sex. CTV News has agreed to protect the full identity of Aziz M. out of concerns for his privacy.
Ko said while the update is an important milestone, his client intends to maintain his challenge against the Health Canada directive, “and the continued discrimination contained in this latest revision.”
“Based on our understanding of the science, there is no scientific justification for screening criteria that continues to discriminate on the basis of sexual activity and sexual orientation, since the testing and quarantine protocols already in place allow sperm banks to detect relevant infections and exclude such donations,” Ko said.
Currently, a Health Canada directive prohibits gay and bisexual men from donating sperm to a sperm bank for general use, unless they’ve been abstinent for three months or are donating to someone they know.
For example, it stops any gay man who is sexually active from donating, even if they are in a long-term monogamous relationship.
Under the “Safety of Sperm and Ova Regulation,” sperm banks operating in Canada must deem these prospective donors “unsuitable,” despite all donations being subject to screening, testing and a six-month quarantine before they can be used.
While the directive does not mention transgender or non-binary donors, the policy also applies to individuals who may not identify as male but would be categorized as men under the directive.
It’s a blanket policy that the Toronto man bringing the lawsuit said made him feel like a “second-class citizen,” and goes to the heart of the many barriers that exist for LGBTQ2S+ Canadians looking to have children.
When CTV News first reported on the lawsuit, Health Canada and various federal ministers said they would be “exploring” a policy change, citing the progress made on blood donation rules.
The update comes following “the consultations held in August 2023 and January 2024,” according to Health Canada.
This is a breaking news story, more to come…
News
Gas prices: Why drivers in Eastern Canada could pay more – CTV News


Drivers in Eastern Canada could see big increases in gas prices because of various factors, especially the higher cost of the summer blend, industry analysts say.
Patrick De Haan, head of petroleum analysis at fuel savings website GasBuddy in Chicago, predicts a big gas hike for the eastern portions of Canada including Ontario, Quebec, Newfoundland and Labrador, New Brunswick and Nova Scotia over the next several days, while some areas in the Maritimes have already seen the increases.
“Unfortunately, for … really a third of Canada, we’re likely to see a big jump in what (motorists) are seeing at the pump,” he said in a video interview with CTVNews.ca. “Gas prices could rise in excess of 10 cents a litre. All of that having to do with yesterday’s switchover to summer gasoline.”
Gas prices may continue to increase for the next week or two, De Haan said. “But I think the end is near for the seasonal increases and we should start to see prices decreasing potentially by May (long weekend).”
Dan McTeague, president of Canadians for Affordable Energy, also forecasts gas price hikes.
Ontario and Quebec will see a 14-cent-per-litre increase overnight Thursday, he said on Wednesday. He predicts the price per litre will rise to $1.79 in cities across Ontario, the highest since Aug. 2, 2022. In Quebec, he expects the price per litre will increase to $1.88.
McTeague attributes this week’s increase to the higher cost of summer blended gasoline.
De Haan, meanwhile, observed the following changes in prices across Canada compared to a week ago:
- Prices in Saskatchewan are flat;
- Manitoba prices are up about a half a penny per litre;
- Alberta is down seven-tenths of a penny per litre;
- P.E.I. is up about 1.2 cents a litre;
- B.C. is up about 2.5 cents a litre;
- Nova Scotia is up three cents a litre;
- Quebec is up 3.5 cents a litre;
- Ontario is up 4.5 cents a litre;
- New Brunswick is up five cents a litre;
- Newfoundland is up seven cents a litre.
Factors behind spikes
“Some gas stations have already raised their price, in essence, but some others may not for the next day or two,” De Haan said. “So over the next several days, the averages will continue to rise as more stations raise their price. … Most of the increase is happening right now in the eastern portions of Canada.”
The summer gas switch will have “just a one-time impact” on gas prices, De Haan said.
More drivers are on the road, creating rising demand for gas as temperatures warm up, and refiners are wrapping up maintenance ahead of the start of the summer driving season. “While they do that maintenance, they’re generally not able to supply as much gasoline into the market,” De Haan explained.
Despite tensions between Iran and Israel, the recent attack has had “little impact” on the price of oil, De Haan said.
“Last week, oil prices did climb to their highest level (in) six months as Iran suggested it was going to attack Israel,” he said. “Now that those attacks have happened and they largely have been unsuccessful, the price of oil is actually declining.”
Third major spike in 2024
Michael Manjuris, professor and chair of global management studies at Toronto Metropolitan University, said the new gas price increase would be the third major spike across Canada since the start of the year.
One factor is the price of crude oil worldwide has risen 15 per cent since Jan. 1, Manjuris said.
The federal carbon tax increase of about 3.3 cents per litre on April 1 is also another reason for the big jolts in gas prices, he added.
Although the switch to summer blend fuels typically happens every year, Manjuris said, it will be more painful economically because it’s on top of the two other major increases this year. “This increase now will cause the overall price of gasoline to be very high,” he said in a video interview with CTVNews.ca. “We haven’t seen these kinds of prices since 2022.”
Manjuris believes gas prices will continue to rise through the summer as global demand for oil begins to grow. “That’s because we’re seeing increased economic activity in China, in the United States and in Europe,” he explained. “When those things all come together, price of crude oil starts to go up. … So I’m predicting that because of demand increasing, price of gasoline in Canada will also go up in the summer months. I’m going to suggest three to five cents a litre will be the peak before it starts to come back down.”
Regional differences
The West Coast and Prairies won’t have any gas price hikes coming soon because they already transitioned to summer gasoline, De Haan said. “So this is something associated with the switchover, which happens last in the eastern parts of Canada,” he explained.
In addition, he said regions have “subtle differences” in their supplies of gasoline.
“Supplies of winter gasoline in the eastern portions of Canada was rather lavish and so discounts were significant,” he said. “But now that the eastern part of Canada is rolling over to relatively tight supplies of summer gasoline, this is something much more impactful. That is other areas of Canada did roll over to summer gasoline, but they did not have necessarily the big discounts that would associate with the big price swing that we’re seeing.”
With files from CP24.com Journalist Codi Wilson
News
For its next trick, Ottawa must unload the $34B Trans Mountain pipeline. It won't be easy – CBC.ca
In her budget speech to the House of Commons on Tuesday, Finance Minister Chrystia Freeland took a moment to celebrate the finishing touch on expansion of the Trans Mountain oil pipeline.
The controversial project has been plagued by delays and massive cost overruns, but Freeland instead focused on its completion, highlighting the: “talented tradespeople and the brilliant engineers who, last Thursday, made the final weld, known as the golden weld, on a great national project.”
For all the difficulties with developing and building TMX, Freeland still faces another major hurdle that is sure to prove contentious — choosing when to sell it, who gets to buy it, and for how much.
An upcoming election and more than $34 billion in construction costs are raising the stakes.
Ottawa bought the project when it was on the verge of falling apart — before there was ever a shovel in the ground — in the face of legal, political and regulatory challenges.
The federal government has long vowed to sell the project (including at least a partial ownership stake to Indigenous groups) once construction was complete. That milestone has now been reached.


But the move will no doubt open a Pandora’s box, says Daniel Béland, the director of the McGill University Institute for the Study of Canada and a professor in the department of political science.
He says any potential deal will face intense scrutiny considering the election is due before the fall of 2025 and, most notably, because the actual sale price is expected to be far lower than the cost to actually build the pipeline.
“They were in a hot spot when they bought it back in 2018. They are still in a hot spot,” said Béland.
How the governing Liberals handle Trans Mountain could impact how voters view the Liberal party’s handling of financial, economic, Indigenous, and environmental issues.
“There’s risk either way. If you sell it really fast, but you sell it at the price that is considered to be quite low, then you might be accused of just getting rid of it for political reasons but not having the interest of taxpayers in mind,” he said.
“But, if you wait and you don’t sell it, then you might be accused of being basically permanently involved or trying to be permanently involved in that sector of the economy in a way that many people, even people who are more conservative, may find inappropriate.”
Deep discount
There has always been interest in buying it, including from Stephen Mason, the managing director of Project Reconciliation, a Calgary-based organization which aims to use a potential ownership stake to benefit Indigenous communities.
Nearly five years ago, Mason walked into then-federal finance minister Bill Morneau’s office in Ottawa and made an offer to purchase Trans Mountain before construction had even begun on its expansion, which will transport more oil from Alberta to the British Columbia coast.
Morneau was interested, he says, but the project wasn’t for sale until the new pipeline was built.
Much has changed since that meeting in July 2019, including the ballooning cost of Trans Mountain to more than $34 billion (compared to an original estimate of about $7.3 billion) and numerous delays in construction.
Mason is still pursuing ownership. He won’t discuss numbers, but suspects Trans Mountain is worth far less than $34 billion.
“My intuition is telling me that it’s going to be a fairly significant writedown,” he said. “I’m not sure the Liberal government wants to get into a public recognition of what the writedown is ahead of the election, but that is just … my speculation.”


New tolls
A critical factor in the timing and price of a potential sale is a dispute over how much oil companies will have to pay to actually use the new pipeline.
Several large oil producers signed long-term contracts to use 80 per cent of the pipeline. However, as construction costs have soared, so too have the tolls that companies will have to pay.
Those companies have balked at the higher rates arguing they shouldn’t have to bear the “extreme magnitude” of construction overruns. The Canada Energy Regulator has scheduled a hearing for September, at the earliest, to resolve the issue.
For now, the regulator has set an interim toll of $11.46 for every barrel of oil moved down the line. That price includes a fixed amount of $10.88 and a variable portion of $0.58. The fixed amount is nearly double what Trans Mountain estimated it would be in 2017.
“There’s no way that you can have tolls high enough on TMX to cover a $34 billion budget,” said Rory Johnston, an energy researcher and founder of the Commodity Context newsletter, who describes the cost overruns on the project compared to the original estimates as “gigantic.”
Lessons could be learned on how the Trans Mountain expansion pipeline was developed and built, says company CFO Mark Maki.
He doesn’t expect the final tolls to be much higher than the interim amount because, otherwise, the pipeline could become too expensive for oil companies to want to use. Based on the interim tolls, Johnston expects the federal government to likely only recover about half of the money it spent to buy and build Trans Mountain.
“There’s no way anyone would pay the full cost of the pipeline because the tolls don’t support it. You’re going to need to discount it. You’re going to need to take a haircut of at least 50 per cent of this pipeline,” he said.
The federal government currently owns the original Trans Mountain pipeline, built in 1953, the now-completed expansion and related facilities including storage tanks and an export terminal.
Potential buyers
The federal government has looked at offering an equity stake to the more than 120 Western Canadian Indigenous communities whose lands are located along the pipeline route, while finding a different buyer to be the majority owner.
Besides Project Reconciliation, other potential buyers include a partnership between the Western Indigenous Pipeline Group (WIPG) and Pembina Pipelines.
The group has the support from about 40 Indigenous communities and hopes to purchase the project within the next year, said Michael Lebourdais, an WIPG director and chief of Whispering Pines/Clinton Indian Band, located near Kamloops, B.C.
Those communities have to live with the environmental risk of a spill, so they should benefit financially from the pipeline, he says.
Pension funds and other institutions could pursue ownership too.
“There will be buyers. I’m not sure that they’ll be willing to pay the full cost of construction but I think there’ll be buyers for sure,” said Jackie Forrest, executive director of the ARC Energy Research Institute.
The federal government will likely highlight the overall economic benefits of the new pipeline and the expected role of Indigenous communities in ownership, experts say, as a way to defend against criticism if the eventual sale price is low.
In her Tuesday speech, Freeland was already promoting the pipeline’s expected financial boost by highlighting the Bank of Canada’s recent estimate that the new Trans Mountain expansion will add one-quarter of a percentage point to Canada’s GDP in the second quarter.
-



 Science7 hours ago
Science7 hours agoJeremy Hansen – The Canadian Encyclopedia
-



 Investment7 hours ago
Investment7 hours agoUK Mulls New Curbs on Outbound Investment Over Security Risks – BNN Bloomberg
-



 Tech7 hours ago
Tech7 hours agoSave $700 Off This 4K Projector at Amazon While You Still Can – CNET
-



 Health21 hours ago
Health21 hours agoSupervised consumption sites urgently needed, says study – Sudbury.com
-


 Tech5 hours ago
Tech5 hours ago'Kingdom Come: Deliverance II' Revealed In Epic New Trailer And It Looks Incredible – Forbes
-



 Sports5 hours ago
Sports5 hours agoAuston Matthews denied 70th goal as depleted Leafs lose last regular-season game – Toronto Sun
-
Real eState6 hours ago
Sick of Your Blue State? These Real Estate Agents Have Just the Place for You. – The New York Times
-
News20 hours ago
Canada's 2024 budget announces 'halal mortgages'. Here's what to know – National Post








