Business
Late-stage COVID-19 vaccine trial on pause due to possible serious side effect. Here's what that means – CBC.ca
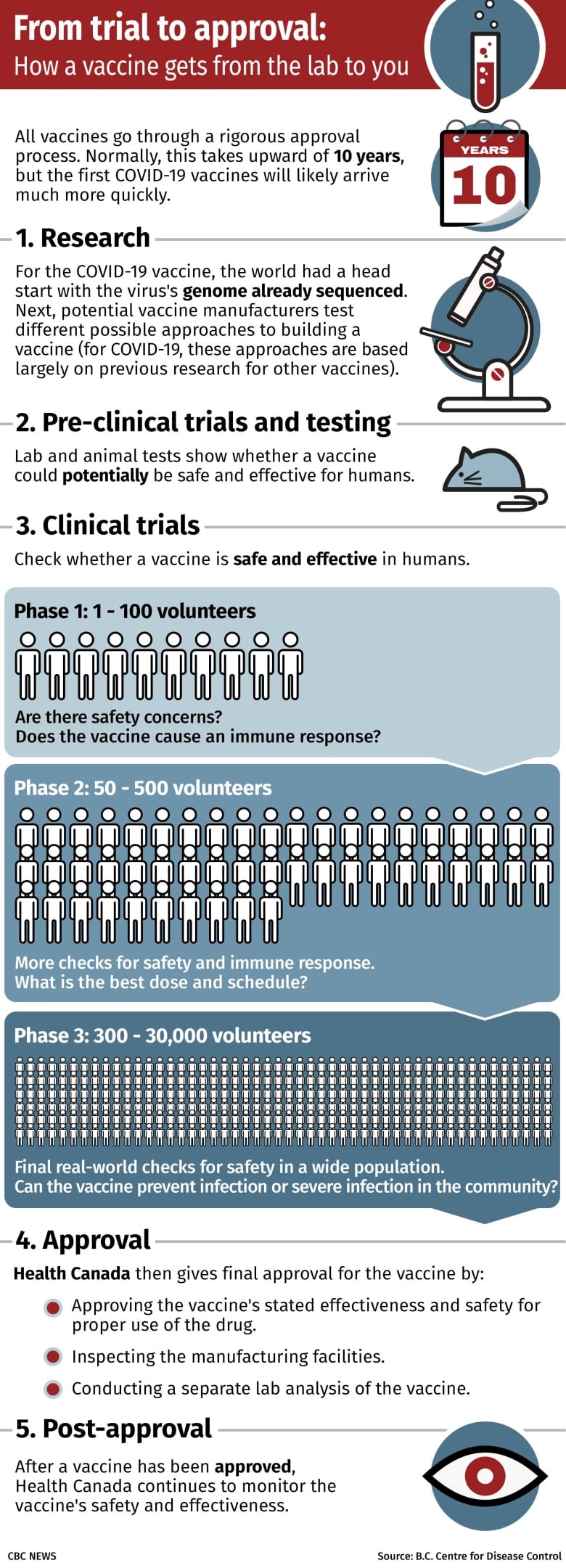
A front-running team in the race to develop a COVID-19 vaccine has put its late-stage trial on hold after a reported “unexplained illness” in one of the trial volunteers. Here’s what that means for the quick development of a COVID-19 vaccine.
What kind of vaccine trial got put on hold?
The trial was a Phase 3 clinical trial for a vaccine being developed by the University of Oxford and pharmaceutical company AstraZeneca.
It’s the largest type of clinical trial, requiring thousands of volunteers, and is the last of three stages of human testing before a vaccine can be approved for use. Its main goals are to:
-
Test the efficacy of the vaccine at preventing the disease compared with a placebo
-
Get a better idea of possible side effects and how often they happen, including rare side effects that might not show up in smaller trials.
The company is running Phase 3 trials involving thousands of people in the United Kingdom and smaller numbers of people in Brazil and South Africa. It is also recruiting 30,000 people in the United States for its largest study.
The vaccine being tested is a non-replicating viral vector vaccine.
The federal government reported on Aug. 31 that it was close to a deal to secure doses of this particular vaccine for Canadians.
Why was the trial suspended?
AstraZeneca reported Tuesday evening that there was a “potentially unexplained illness” in one of its trials in the U.K.
That triggered a “standard review process,” intended to ensure safety when that happens.
While the trial is suspended, the incident will be investigated by independent reviewers not involved in the trial itself.
What kind of illness was it?
AstraZeneca said Wednesday that the patient had neurological symptoms associated with a spinal inflammatory disorder called transverse myelitis, but a final diagnosis was still pending as more tests are carried out, Reuters reported.
That involves localized inflammation of the spinal cord, which can cause symptoms such as weakness, loss of sensation or even paralysis of the arms and legs. It can be caused by autoimmune diseases, viral, bacterial or fungal infections or parasites, but it has also been reported as potentially a rare side effect of vaccinations for diseases such as influenza or rubella.
However, researchers who have studied it note that it’s difficult to confirm or exclude the link between the disease and vaccination, since it can occur coincidentally due to other causes after vaccination.
WATCH | Infectious disease specialist explains suspension of trial:
What is the goal of the review?
It will try to determine whether the illness was related to the vaccine.
Because trials like this are typically double-blinded, the researchers don’t know whether a given volunteer received the vaccine or a placebo. That’s one of the reasons why the review needs to be conducted by an independent committee that is not doing other analyses in the study.
Even if the volunteer received the vaccine, the timing of the illness could still be coincidental and unrelated to the vaccine.
Dr. Sumon Chakrabarti, an infectious disease specialist at Trillium Health Partners in Toronto, told CBC News Network that if the patient does have transverse myelitis, he or she will likely be tested for different types of infections to see if a cause can be determined.
“I’ve seen many of these cases myself, and we often come up with viral causes,” he said.
If that happens, the review may be able to rule out the vaccine as the cause and allow the trial to resume.
WATCH | Pausing massive vaccine trial isn’t routine, respirologist says:
How often do pauses like this happen?
On the one hand, they’re not triggered by “mild” side effects, and there haven’t been any publicized for any COVID-19 vaccine trials so far, despite the large number underway. However, AstraZeneca disclosed Wednesday that it had briefly paused a COVID-19 vaccine trial in July after a study volunteer was found to have multiple sclerosis. An independent review panel concluded the illness was not related to the vaccine.
Dr. Samir Gupta, associate professor of medicine at the University of Toronto, said that “it’s not a routine thing to stop a massive trial mid-course like this.”
But on the other hand, it’s not unexpected, given the size of the trial, said Dr. Michael Gardam, an infectious disease specialist at Women’s College Hospital in Toronto.
“I would argue for probably every vaccine that’s ever come to market, there’s been an event like this,” Gardam said.
“When you’re giving vaccine to tens of thousands of people, something’s going to happen to one of them. And chances are it’s happenstance … it’s not linked to the vaccine. But each time, you have to investigate it.”
WATCH | How COVID-19 vaccines are being created quickly and safely:
Will the pause slow down development of a vaccine?
“Not necessarily, it depends on what they find when they do the investigation,” British Health Secretary Matt Hancock said on Wednesday.
Gardam said he doesn’t think it will cause a significant delay.
Investigators will try to figure out “a reasonable explanation” for the cause of the illness, Gardam said, which may take some time.
A pause occurred during the Phase 1 trial of a Canadian-made Ebola vaccine in 2014 after several volunteers reported joint pain. An investigation found that the side effect was likely caused by the vaccine, and the study resumed three weeks later with a lower dose.
In this case, Gardam said he thinks it will be hard to draw any conclusion based on one illness and that the University of Oxford researchers will be able to “quite quickly get back up and running again.”
However, they will need to collect more data to see if others show similar illnesses. If that happens, he said, “then that’s a completely different story.”
WATCH | When will a COVID-19 vaccine be ready?:
How worried should we be about this pause?
If it turns out that this is a potential adverse effect of this vaccine, “that would obviously be a substantial showstopper for this vaccine,” said Dr. Andrew Morris, an infectious disease specialist at Sinai Health, the University Health Network and the University of Toronto.
He’s concerned there wouldn’t be access to the vaccine, which is in advanced stages of development. It’s also one that many countries are pinning their hopes on, with substantial investment and three billion doses reserved by governments around the world and by the COVAX Facility, which aims to provide access to more than 70 per cent of the world’s population.
Morris said he’s also concerned that the media coverage will discourage people from enrolling in vaccine studies or increase anti-vaccination hype.
“Any step back is really a setback for all of us,” he said.
But at the same time, researchers such as Gardam say in some ways, the pause should ease people’s concerns, as it shows that the system is working and highlights the importance of Phase 3 clinical trials to ensure the safety of vaccines.
“This in and of itself isn’t a big deal,” he said. “This is what is supposed to happen…. This gives me some comfort.
“The fact that this has been stopped appropriately, it’ll be investigated. We’ll learn about it and then presumably the trial will start up again. That’s exactly what’s supposed to happen.”
Business
Tesla Promises Cheap EVs by 2025 | OilPrice.com – OilPrice.com


Tesla has promised to start selling cheaper models next year, days after a Reuters report revealed that the company had shelved its plans for an all-new Tesla that would cost only $25,000.
The news that Tesla was scrapping the Model 2 came amid a drop in sales and profits, and a decision to slash a tenth of the company’s global workforce. Reuters also noted increased competition from Chinese EV makers.
Tesla’s deliveries slumped in the first quarter for the first annual drop since the start of the pandemic in 2020, missing analyst forecasts by a mile in a sign that even price cuts haven’t been able to stave off an increasingly heated competition on the EV market.
Profits dropped by 50%, disappointing investors and leading to a slump in the company’s share prices, which made any good news urgently needed. Tesla delivered: it said it would bring forward the date for the release of new, lower-cost models. These would be produced on its existing platform and rolled out in the second half of 2025, per the BBC.
Reuters cited the company as warning that this change of plans could “result in achieving less cost reduction than previously expected,” however. This suggests the price tag of the new models is unlikely to be as small as the $25,000 promised for the Model 2.
The decision is based on a substantially reduced risk appetite in Tesla’s management, likely affected by the recent financial results and the intensifying competition with Chinese EV makers. Shelving the Model 2 and opting instead for cars to be produced on existing manufacturing lines is the safer move in these “uncertain times”, per the company.
Tesla is also cutting prices, as many other EV makers are doing amid a palpable decline in sales in key markets such as Europe, where the phaseout of subsidies has hit demand for EVs seriously. The cut is of about $2,000 on all models that Tesla currently sells.
By Charles Kennedy for Oilprice.com
More Top Reads From Oilprice.com:
Business
Why the Bank of Canada decided to hold interest rates in April – Financial Post
Article content
Divisions within the Bank of Canada over the timing of a much-anticipated cut to its key overnight interest rate stem from concerns of some members of the central bank’s governing council that progress on taming inflation could stall in the face of stronger domestic demand — or even pick up again in the event of “new surprises.”
“Some members emphasized that, with the economy performing well, the risk had diminished that restrictive monetary policy would slow the economy more than necessary to return inflation to target,” according to a summary of deliberations for the April 10 rate decision that were published Wednesday. “They felt more reassurance was needed to reduce the risk that the downward progress on core inflation would stall, and to avoid jeopardizing the progress made thus far.”
Article content
Others argued that there were additional risks from keeping monetary policy too tight in light of progress already made to tame inflation, which had come down “significantly” across most goods and services.
Some pointed out that the distribution of inflation rates across components of the consumer price index had approached normal, despite outsized price increases and decreases in certain components.
“Coupled with indicators that the economy was in excess supply and with a base case projection showing the output gap starting to close only next year, they felt there was a risk of keeping monetary policy more restrictive than needed.”
In the end, though, the central bankers agreed to hold the rate at five per cent because inflation remained too high and there were still upside risks to the outlook, albeit “less acute” than in the past couple of years.
Despite the “diversity of views” about when conditions will warrant cutting the interest rate, central bank officials agreed that monetary policy easing would probably be gradual, given risks to the outlook and the slow path for returning inflation to target, according to the summary of deliberations.
Article content
They considered a number of potential risks to the outlook for economic growth and inflation, including housing and immigration, according to summary of deliberations.
The central bankers discussed the risk that housing market activity could accelerate and further boost shelter prices and acknowledged that easing monetary policy could increase the likelihood of this risk materializing. They concluded that their focus on measures such as CPI-trim, which strips out extreme movements in price changes, allowed them to effectively look through mortgage interest costs while capturing other shelter prices such as rent that are more reflective of supply and demand in housing.
Recommended from Editorial
They also agreed to keep a close eye on immigration in the coming quarters due to uncertainty around recent announcements by the federal government.
“The projection incorporated continued strong population growth in the first half of 2024 followed by much softer growth, in line with the federal government’s target for reducing the share of non-permanent residents,” the summary said. “But details of how these plans will be implemented had not been announced. Governing council recognized that there was some uncertainty about future population growth and agreed it would be important to update the population forecast each quarter.”
• Email: bshecter@nationalpost.com
Bookmark our website and support our journalism: Don’t miss the business news you need to know — add financialpost.com to your bookmarks and sign up for our newsletters here.
Share this article in your social network
Business
Meta shares sink after it reveals spending plans – BBC.com


Shares in US tech giant Meta have sunk in US after-hours trading despite better-than-expected earnings.
The Facebook and Instagram owner said expenses would be higher this year as it spends heavily on artificial intelligence (AI).
Its shares fell more than 15% after it said it expected to spend billions of dollars more than it had previously predicted in 2024.
Meta has been updating its ad-buying products with AI tools to boost earnings growth.
It has also been introducing more AI features on its social media platforms such as chat assistants.
The firm said it now expected to spend between $35bn and $40bn, (£28bn-32bn) in 2024, up from an earlier prediction of $30-$37bn.
Its shares fell despite it beating expectations on its earnings.
First quarter revenue rose 27% to $36.46bn, while analysts had expected earnings of $36.16bn.
Sophie Lund-Yates, lead equity analyst at Hargreaves Lansdown, said its spending plans were “aggressive”.
She said Meta’s “substantial investment” in AI has helped it get people to spend time on its platforms, so advertisers are willing to spend more money “in a time when digital advertising uncertainty remains rife”.
More than 50 countries are due to have elections this year, she said, “which hugely increases uncertainty” and can spook advertisers.
She added that Meta’s “fortunes are probably also being bolstered by TikTok’s uncertain future in the US”.
Meta’s rival has said it will fight an “unconstitutional” law that could result in TikTok being sold or banned in the US.
President Biden has signed into law a bill which gives the social media platform’s Chinese owner, ByteDance, nine months to sell off the app or it will be blocked in the US.
Ms Lund-Yates said that “looking further ahead, the biggest risk [for Meta] remains regulatory”.
Last year, Meta was fined €1.2bn (£1bn) by Ireland’s data authorities for mishandling people’s data when transferring it between Europe and the US.
And in February of this year, Meta chief executive Mark Zuckerberg faced blistering criticism from US lawmakers and was pushed to apologise to families of victims of child sexual exploitation.
Ms Lund-Yates added that the firm has “more than enough resources to throw at legal challenges, but that doesn’t rule out the risks of ups and downs in market sentiment”.
-



 Politics23 hours ago
Politics23 hours agoOpinion: Fear the politicization of pensions, no matter the politician
-


 Science23 hours ago
Science23 hours agoNASA Celebrates As 1977’s Voyager 1 Phones Home At Last
-



 Politics22 hours ago
Politics22 hours agoPecker’s Trump Trial Testimony Is a Lesson in Power Politics
-
Media16 hours ago
B.C. online harms bill on hold after deal with social media firms
-
Media22 hours ago
B.C. puts online harms bill on hold after agreement with social media companies
-
Business22 hours ago
Oil Firms Doubtful Trans Mountain Pipeline Will Start Full Service by May 1st
-
Media21 hours ago
Trump poised to clinch US$1.3-billion social media company stock award
-
Art24 hours ago
Turner Prize: Shortlisted artist showcases Scottish Sikh community















