PWD water sampling in the ISS
On January 8, 2021, an astronaut collected 350 mL of potable water from the PWD on the United States On-orbit Segment (USOS) of the ISS into NASA’s Post-Flight Analysis Bag. It is made of fluorinated ethylene propylene (FEP) with polypropylene female Luer lock ports and is used for water sample collection and analyses by NASA. The ISS Potable Water Sampling Bag was loaded onto the SpaceX CRS-21 (SpX-21) cargo dragon capsule, which splashed down in the Gulf of Mexico on January 14, 2021 and was transported to NASA’s Kennedy Space Center (KSC) at room temperature. After arrival at the KSC, the bag was stored at 4 °C and transported from the KSC to the Tsukuba Space Center, JAXA, Japan, arriving on January 21, 2021. Ground control water was prepared in our laboratory by collecting ultrapure water in the same bag at the same time as the PWD water sampling and was stored under the same conditions until the measurement.
Total direct counting and CFU counting
Bacteria present in the PWD water were filtered through a black polycarbonate filter (pore size of 0.2 µm, ADVANTEC). Filters were rinsed twice with bacterium-free distilled water. Then, 1 µg/ml of DAPI in distilled water or 150 µg/ml of 6-CFDA in phosphate buffer (0.3 M phosphate (pH 8.5), 15% NaCl, 1.5 mM EDTA) was applied to the filters and incubated for 3 min at room temperature under dark conditions. Filters were rinsed twice, mounted on glass microscope slides with non-fluorescent immersion oil, and examined using an epifluorescence microscope (DM2500, Leica Microsystems) with an oil immersion objective. The number of CFU was determined by spreading diluted ISS potable water on BD TSA and BD BBL R2A agar (Becton, Dickinson and Company), which were incubated for one week before counting at 30 °C and 22 °C, respectively.
MALDI-TOF MS spectrometry for identification of isolated bacteria
A MALDI-TOF MS was used for direct identification of bacteria grown on TSA and R2A agar24. 18 and 20 colonies isolated from TSA and R2A were smeared onto the MALDI MSP 96 polished steel target plate. One microliter of 70% formic acid was deposited onto each sample spot and allowed to dry. Then, 1 µL of matrix solution (α-cyano-4-hydroxycinnamic acid (Bruker Daltonics) dissolved in 50% acetonitrile, 47.5% water, and 2.5% trifluoroacetic acid) was applied onto each sample spot and allowed to dry. Bruker Bacterial Test Standard, Escherichia coli DH5α was used for calibration. Measurements were performed with a Microflex mass spectrometer (Bruker Daltonics) using the flexControl software version 3.4. Mass spectra were acquired in a linear positive extraction mode ranging from 2000 to 20,000 Da. The spectra were analyzed using the MALDI Biotyper 3.1 software with Bruker library BDAL Ver. 6 and Filamentous Fungi Library Ver. 1.0 (Bruker Daltonics). A manufacturer-recommended cutoff score was used for identification (Supplementary Table 2).
Bacterial cell counting using a biofluorescent particle counter
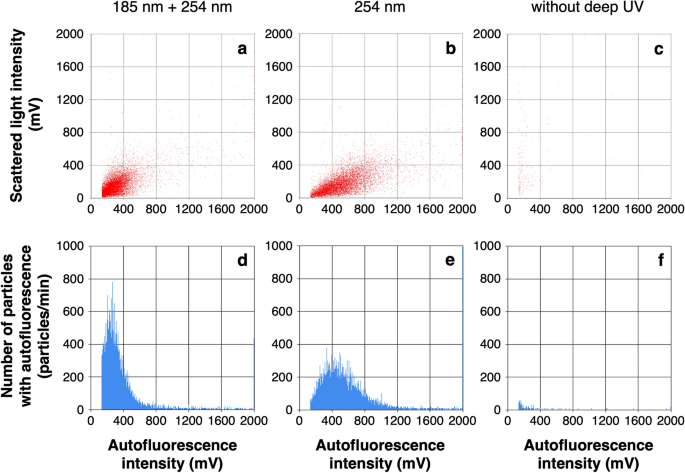
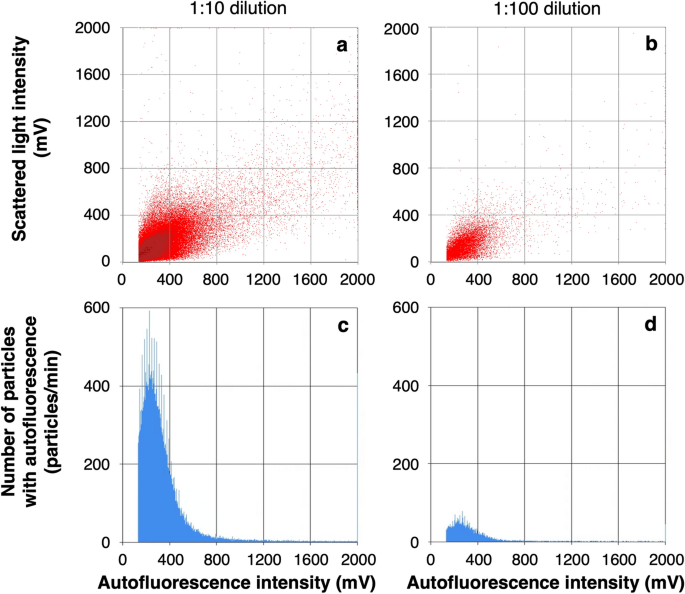
A commercially available biofluorescent particle counter (XL-10BT1, Rion Co. Ltd.) was used in this study. It had two detectors. One was a photodiode for measuring the scattered light intensity, which indicates particle size. The other was a photomultiplier tube for measuring the autofluorescence intensity, which is an indicator of the physiological activity of bacteria. This allowed the scattered light intensity and the autofluorescence intensity to be measured simultaneously.
We developed a protocol to measure bacterial cells without staining using the biofluorescent particle counter, identifying particles emitting autofluorescence from flavin and counting them as bacterial cells. Flavin is a ubiquitous pigment in bacterial cells, which emits autofluorescence at 510 nm when irradiated with 405 nm excitation light25. The amount of intracellular flavin is closely related to the physiological activity of bacteria25, and thus bacteria with low physiological activity may not be detected due to low autofluorescence intensities. To solve this problem, we irradiated particles with deep UV irradiation, at a wavelength of 254 nm or two wavelengths of 185 nm and 254 nm, to oxidize flavin and enhance autofluorescence intensity immediately before the measurement under irradiation at 405 nm, which was carried out using a deep UV irradiation device (XL-28A, RION Co. Ltd.) equipped with a low-pressure mercury lamp. Since deep UV irradiation is known to degrade organic carbon26,27, the deep UV irradiation incorporated in our bacterial cell counting protocol was also used for reducing signals from dissolved organic carbon in the PWD water. Particle counting was performed at a flow rate of 10 mL/min and a measurement time of 60 s. Deep UV irradiation dose was over 1000 mJ/cm2. Before measuring bacterial cells, ultrapure water was used to set a cutoff threshold for electrical noise derived from the biofluorescent particle counter, which was determined as 133 mV. To exclude particles with extremely high autofluorescence intensity from being counted as bacteria, upper threshold was defined at 1200 mV when counting bacterial cells.
DNA extraction
Bacterial cells present in 50 mL of the PWD water were trapped onto an autoclaved polycarbonate membrane filter (pore size of 0.2 μm; Advantec, Tokyo, Japan). Bacterial DNA was extracted with the method described in Ichijo et al.2. The DNA was finally eluted with 50 µL of TE buffer.
Amplicon sequencing of bacterial 16S rRNA gene by ONT MinION platform
Amplicon sequencing targeting the full length of the 16S rRNA gene was performed using MinION equipped with R9.4.1 flow cell (Oxford Nanopore Technologies, Oxford, UK). A 16S rRNA sequencing library was constructed from 10 µL of extracted DNA using the 16S barcoding kit (Oxford Nanopore Technologies). The library construction was performed according to the manufacturer’s instructions except that DNA amplification was carried out using KAPA HiFi HotStart ReadyMix (KAPA Biosystems, MA, USA) with the following thermal cycling conditions: 2 min at 95 °C, 25 cycles of 20 s at 98 °C, 30 s at 60 °C, and 2 min at 72 °C, and 5 min at 72 °C. Sequencing was carried out with Oxford Nanopore’s MinKNOW software and basecalls were performed using Guppy (v. 4.3.4) in fast mode using the config file dna_r9.4.1_450bps_fast.cfg. Generated FASTQ files were further analyzed for taxonomic classification using the cloud-based EPI2ME FASTQ 16S workflow with a quality score ≥ 7 for quality filtering.
Amplicon sequencing of bacterial 16S rRNA gene by Illumina MiSeq and iSeq platforms
Amplicon sequencing targeting the 16S rRNA gene V4 region was performed using the 300-bp paired-end MiSeq platform with MiSeq Reagent Kit v2 and 150-bp paired-end iSeq platform with iSeq 100 i1 Reagent (Illumina, CA, USA). A two-step PCR was performed to construct paired-end libraries. In the first PCR, the V4 region of the prokaryotic 16S rRNA gene was amplified from the DNA sample using F515 and R806 primers28 with the Illumina overhang adapters. The first PCR reactions were carried out in triplicate in 12 µL reaction volume containing 3 µL of the template and 0.6 µM each of forward and reverse primers in 1 × KAPA HiFi HotStart ReadyMix (KAPA Biosystems) using thermal cycling of 2 min at 95 °C, 35 cycles of 20 s at 98 °C, 15 s at 60 °C, and 30 s at 72 °C, and 5 min at 72 °C. The triplicate PCR products were pooled and purified using Agencourt AMPure XP (Beckman Coulter, CA, USA) according to the manufacturer’s instructions. A second PCR (12 cycles) was performed to attach dual indices and Illumina sequencing adapters to the purified first PCR products using Nextera XT Index Kit v2 Set C (Illumina). Finally, the indexed amplicons were purified by electrophoresis using E-Gel SizeSelect II Agarose Gels (Thermo Fisher Scientific, MA, USA). The DNA concentrations of the indexed amplicons were quantified by a Qubit 4 Fluorometer using dsDNA HS Assay Kit (Thermo Fisher Scientific) and pooled in equal amounts for library construction. The library was diluted to 1 nM and spiked with 20% PhiX Control v3 (Illumina), then diluted to 50 pM, loaded into MiSeq and iSeq cartridges, and sequenced according to the manufacturer’s instructions.
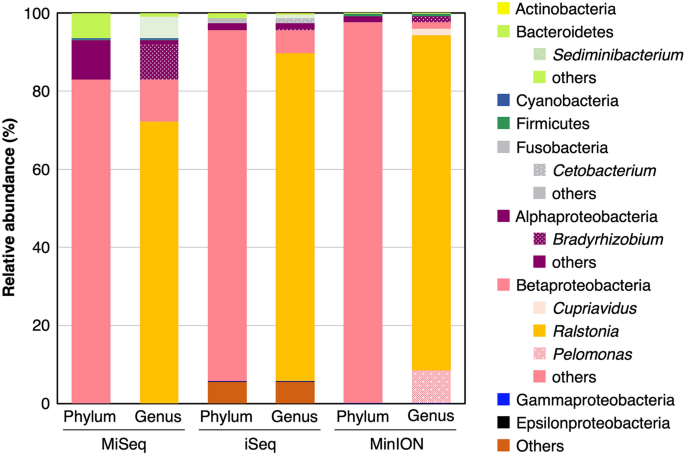
FASTQ files generated from MiSeq and iSeq sequencing were analyzed separately using QIIME 2 pipeline29 (v. 2021.8). Denoising sequences, merging paired-end reads, and chimera filtering were performed using the qiime dada2 denoise-paired method of the DADA230,31 plugin in QIIME 2, where parameters were adjusted from defaults both for MiSeq and iSeq data as follows: –p-trim-left-f 19, –p-trim-left-r 20, –p-max-ee-f 1.0, and –p-max-ee-r 1.0. In addition to these adjustments, the parameters –p-trunc-len-f and –p-trunc-len-r were adjusted to 263 and 226 for the MiSeq data to truncate forward and reverse reads at the position with a median quality score lower than 30. As to the iSeq data, forward and reverse reads were not truncated. The parameter –p-min-overlap, which controls the minimum overlap required for merging the paired reads, was reduced from the default (12) to 5 to enable merging. The yielded non-chimeric sequences, called Amplicon Sequence Variants (ASVs), were assigned to taxonomic groups using ‘qiime feature-classifier classify-sklearn’31 using a naïve Bayes classifier pre-trained on the Silva 138 99% database32 for 515F/806R region of 16S rRNA (silva-138-99-515-806-nb-classifier.qza) with a confidence threshold of 0.985.