Viral fragments of bird flu have been identified in samples of milk taken from grocery store shelves in the United States, a finding that does not necessarily suggest a threat to human health but indicates the avian flu virus is more widespread among dairy herds than previously thought, according to two public health officials and a public health expert who was briefed on the issue.
Health
Learning how the coronavirus affects the body offers clues to fight COVID-19 – CBC.ca

This is an excerpt from Second Opinion, a weekly roundup of eclectic and under-the-radar health and medical science news emailed to subscribers every Saturday morning. If you haven’t subscribed yet, you can do that by clicking here.
The devastating damage that the novel coronavirus inflicts on the human body can set off inflammatory havoc. As we learn more, doctors are gaining clues to hopefully prevent deaths and improve treatment.
Last December, COVID-19 entered the world stage as a flu-like illness causing fever, dry cough and a sore throat.
Since then, the list of how the illness can present has expanded, and expanded again, to include gastrointestinal symptoms like diarrhea, general aches, loss of taste and smell and serious blood-clotting problems, among others.
Of the more than five million infections globally so far, 2.4 million have recovered.
Most infected people have so few symptoms they are better off at home. The minority of serious infections in patients — mainly those over 65, though no age group is left unscathed — can confound health professionals caring for them.
The illness can worsen to a severe stage called Acute Respiratory Distress Syndrome, which includes severe lung inflammation and damage. These are often the patients who are admitted to intensive care units and need life support such as ventilation.
Dr. Lynora Saxinger, an infectious disease physician at the University of Alberta, co-chairs a provincial scientific advisory board reviewing how COVID-19 manifests and what it means for reducing transmission and extending treatment beyond current care measures.
“The landscape shifts really quickly,” Saxinger said. “We just want to make sure that we’re not missing [what] could be spreading, because that’s where we’re going to run into trouble.”
Not typical clots
As initial anecdotes about inflammatory-like effects such as blood-clotting complications mounted into a clearer signal for caution, clinicians adapted their care while scientists worked to understand why it happens.
Now, Saxinger said there’s more evidence of clotting damage in both large and small blood vessels. “This virus is doing different things in the body.”
Experts say some of these inflammatory effects look to be unique to this particular coronavirus, which is known as SARS CoV-2.
Dr. Zain Chagla, an associate professor of infectious disease at McMaster University in Hamilton, Ont., said the wide extent of clotting with this virus differs from other infections, including from the deadly SARS and MERS coronaviruses. With COVID-19, the clots occur in veins in the legs and lungs, as well as in arterial ones that cause strokes and can lead surgeons to resort to amputating a patient’s limbs.
Medical researchers have also found tiny clots that damaged tissue throughout the body in hospitalized patients and in autopsies.
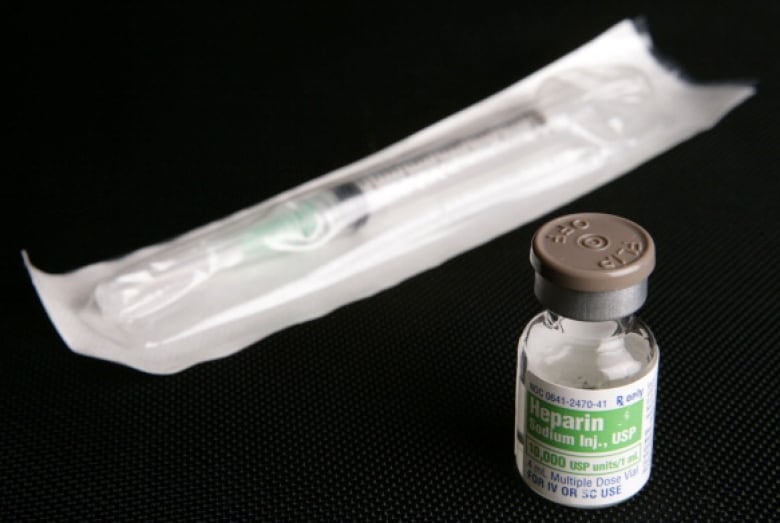
Chagla said this means that “from a therapeutic standpoint,” it might be better to give patients a low dose of heparin, an anticoagulant or blood thinner. It’s often used before surgery and in a variety of medical conditions to prevent and treat clots.
Clinical trial researchers are also exploring the use of high-dose anti-coagulants in carefully selected patients, Chagla said.
This week, Health Minister Patty Hajdu announced an accelerated path for clinical trials to help find answers to urgent COVID-19 diagnosis, treatment, mitigation or prevention questions while keeping patients safe.
On Friday, Montserrat Puig of the U.S. Food and Drug Administration and her team published what they called a road map for effective treatment of COVID-19, based on both repurposing existing approved drugs as well as those still under development.
The review, published in Frontiers in Immunology, unravels factors leading to the “cytokine storm” that can rampage in people with severe COVID-19. Cytokines are small molecules released by the body’s immune system to co-ordinate response against an infection or injury, ranging from a mild fever to suspected deaths in the 1918 flu pandemic.

Scientists are still working to understand the key events in cells, tissues and the body’s immune system that tips the balance from a normal, protective, “hey, come help” call for reinforcements to an unnecessary, four-alarm call that leads to a life-threatening overreaction.
Puig wrote that potential drugs include those that could block the virus from entering our cells in the first place, antivirals to stop the virus from making copies of itself and therapies called monoclonal antibodies that dampen the haywire response from cytokines.
Inflammatory storm unleashed
People who develop symptoms of COVID-19 do so within 14 days, and it mostly occurs about five days after exposure.


Saxinger said when patients struggle with congested lungs and poor blood pressure control, it’s often a manifestation of lung inflammation in response to the infection.
She said there’s also an arc to the story of how the disease marches through the body from initial infection to damage to recovery or death.
“The initial infection triggers this body-wide response that is devastating,” Saxinger said. “Then, when the infection itself might be coming under control, it’s almost like you unleash this storm of immune reactivity and inflammation.”
Once the storm is set off, doctors say treating the infection itself is unlikely to help much.
So, what could help? As physicians report more symptoms, scientists working in parallel are exploring why and how the virus replicates in some tissues and organs so well.
Matthew Miller, an associate professor of infectious disease and immunology at McMaster, is following the scientific advances.
The virus seems to use a receptor called ACE2 to enter human cells. Miller said many groups of researchers are working to understand what cells in our body have active proteins where the virus might be able to replicate and cause disease.
“Knowing what cells a virus is capable of infecting is really important, because it can help us anticipate what types of diseases or what types of symptoms it might cause,” Miller said.
It’s thought that the infectious dose a person is exposed to, as well as minute, genetic differences in the individual and whether they have underlying health conditions (like heart disease or diabetes) all play a role in how COVID-19 manifests.
Understand virus to guide reopening
For now, medical researchers are exploring how ramping up a beneficial aspect of the immune response that cells normally use to kill off a virus could be complemented with “immune modulators” to tamp down overreactions. It’s a delicate balance and timing is key.
Miller said as we learn more about the unique features of SARS-CoV-2, governments and public health officials have been forced to “learn on the fly” and adapt pandemic plans built for a different respiratory infection: influenza, commonly called flu.
“One of the areas that this pandemic has really brought to light is that there’s not enough focus on prevention control measures,” he said.
Countries imposed and eased lockdowns without a firm grasp on what measures work best for this particular virus, leading to differences across the globe and shifting recommendations on wearing masks or physical distancing.
“I think we’re all learning that we don’t understand nearly as well as we should,” Miller said.
WATCH | Why we should expect waves of COVID-19:
Public health basics like staying home when sick, handwashing and cough etiquette apply to all respiratory pathogens. It’s the specifics that are still a work in progress.
For Saxinger, these knowledge gaps mean that understanding COVID-19 will be a long-term effort.
“It’s not just going to be a one, we’re done,” Saxinger said. “We are all going to have to figure out the best way to manage people and try to give them the best outcomes possible.”
To read the entire Second Opinion newsletter every Saturday morning, subscribe by clicking here.
Health
Canada Falling Short in Adult Vaccination Rates – VOCM


Canada is about where it should be when it comes to childhood vaccines, but for adult vaccinations it’s a different story.
Dr. Vivien Brown of Immunize Canada says the overall population should have rates of between 80 and 90 per cent for most vaccines, but that is not the case.
She says most children are in that range but not for adult vaccines and ultimately the most at-risk populations are not being reached.
She says the population is under immunized for conditions such as pneumonia, shingles, tetanus, and pertussis.
Brown wants people to talk with their family physician or pharmacist to see if they are up-to-date on vaccines, and to get caught up because many are “killer diseases.”
Health
Bird flu virus found in grocery milk as officials say supply still safe – The Washington Post

The Food and Drug Administration said Tuesday that it had been testing milk samples throughout the dairy production process and confirmed the detection of viral particles “in some of the samples,” but it declined to provide details.
The presence of genetic fragments of the virus in milk is not unexpected. Pasteurization typically works to inactivate pathogens, said Jennifer Nuzzo, director of the Pandemic Center at the Brown University School of Public Health. It generally does not remove genetic material, Nuzzo said, but typically renders pathogens unable to cause harm to people.
The greater concern, however, “is that it’s showing up in a lot more samples, meaning the infection is more widespread in dairy herds than we thought,” said one public health official, who spoke on the condition of anonymity to share information not yet made public.
In a four-page statement, the FDA said some of the samples collected have “indicated the presence” of the bird flu virus based on testing that detects viral particles but does not distinguish whether they are active or dead. The finding of virus “does not mean that the sample contains an intact, infectious pathogen,” the agency’s statement said.
Additional laboratory testing is underway to grow the virus in cells and in fertilized eggs, the latter being the “gold standard” for sensitive detection of active, infectious virus, the FDA said. “Importantly, additional testing is required to determine whether intact pathogen is still present and if it remains infectious, which determines whether there is any risk of illness associated with consuming the product,” the FDA statement said.
FDA officials said results are expected in the next few days to weeks.
“To date, we have seen nothing that would change our assessment that the commercial milk supply is safe,” the agency said in its statement.
Officials and experts did not have additional details about the number of milk samples that were positive for particles of bird flu or where the samples originated.
Although this strain of avian flu has been circulating for more than 20 years, its leap into cows is of substantial concern, surprising even longtime observers of the virus. More than two dozen livestock herds in at least eight states have been infected with avian flu since March 25, prompting investigations by federal and state officials.
For weeks, key federal agencies have expressed confidence in the safety of the commercial milk supply, including pasteurized products sold at grocery stores. The FDA has highlighted data showing pasteurization inactivates other viruses and pointed to studies showing that the pasteurization process for eggs — which occurs at a lower temperature than what is used for milk — deactivates the highly pathogenic avian influenza.
The International Dairy Foods Association, which represents the nation’s dairy manufacturing and marketing industry, said that viral fragments are “nothing more than evidence that the virus is dead.”
“Milk and milk products produced and processed in the United States are among the safest in the world,” spokesman Matt Herrick wrote in an email, adding that “viral fragments are simply indicative of pasteurization doing its job effectively and protecting our commercial milk supply.”
In recent weeks, multiple experts expressed confidence that the pasteurization process ensures there is no threat to the safety of the nation’s milk supply but said the federal government should still perform tests to confirm that is the case.
Flu is a “fairly wimpy virus,” meaning it is “fairly readily inactivated,” said Richard J. Webby, a virologist at St Jude Children’s Research Hospital. “But that’s something that has to be tested.”
One case of avian flu has been reported in a Texas farmworker in recent weeks, only the second human case ever of bird flu in the United States.
So far, the virus has not acquired the ability to spread efficiently in people.
But as it is able to jump from animal to animal, prospects increase for it mutating to cause sustained person-to-person transmission — a development that could fuel a pandemic.
State health officials have tested 23 people with flu-like symptoms, but only the dairy worker in Texas has tested positive during the current outbreak. Ongoing surveillance of emergency department visits and flu tests in regions where bird flu has been detected has not flagged unusual trends in flu-like illnesses, or eye inflammation, the only symptom experienced by the dairy worker, according to officials at the Centers for Disease Control and Prevention, who say the risk to the general public of bird flu remains low.
The lack of more human cases is a good sign, health officials say.
The key to containing the outbreak resides in livestock herds. Testing of cows is voluntary. U.S. Department of Agriculture protocols restrict testing to cows with specific symptoms and limits the number of tests per farm.
Health
Avian influenza spread: WHO gives public health warning as FDA calms food safety concerns – Food Ingredients First


23 April 2024 — The World Health Organization (WHO) has warned that the ongoing spread of avian influenza poses a “significant public health concern” and urged health authorities, especially in the US, to closely monitor infections in cows. However, the US FDA maintains that the virus is not currently a concern to consumer health and downplayed its impact on commercial milk production.
Earlier this month, the largest producer of fresh eggs in the US halted production at a Texas plant after bird flu was detected in its chickens. Cal-Maine Foods said that about 3.6% of its total flock was destroyed after the infection.
However, the virus, also known as H5N1, has now been found in at least 26 dairy herds across eight US states, marking the first time this strain of bird flu has been detected in cattle, according to officials.
At least 21 states have restricted cattle importations from states where the virus is known to have infected dairy cows.
The US Department of Agriculture’s Animal and Plant Health Inspection Service strongly recommends minimizing the movement of cattle, but has not issued federal quarantine orders.
Public health threat
The US Centers for Disease Control and Prevention (CDC) confirmed this month that a dairy worker in Texas, who reportedly had exposure to dairy cattle presumed to have had avian influenza, contracted the virus and is now recovering.
“This infection does not change the H5N1 bird flu human health risk assessment for the US general public, which CDC considers to be low,” the agency said in a press release, while acknowledging that people who come into more frequent contact with possibly infected birds or other mammals have a higher risk.
Meanwhile, WHO’s chief scientist, Dr. Jeremy Farrar, told reporters recently in Geneva, Switzerland, that H5N1 has had an “extremely high” mortality rate among the several hundred people known to have been infected with it to date.


“H5N1 is an influenza infection, predominantly started in poultry and ducks and has spread effectively over the course of the last one or two years to become a global zoonotic — animal — pandemic,” said Farrar.
“The great concern, of course, is that in doing so and infecting ducks and chickens — but now increasingly mammals — the virus now evolves and develops the ability to infect humans.
“And then critically, the ability to go from human-to-human transmission.”
Concerns with cattle
US health officials have stressed that bird flu’s risk to the public is low, and the country’s food supply remains safe and stable.
“At this time, there continues to be no concern that this circumstance poses a risk to consumer health or that it affects the safety of the interstate commercial milk supply,” the FDA said in a statement.
According to officials, farmers are being urged to test cows that show symptoms of infection and separate them from the herd, where they usually recover within two weeks.
US producers are not permitted to sell milk from sick cows, while milk sold across state lines must be pasteurized or heat-treated to kill viruses, including influenza.

“We firmly believe that pasteurization provides a safe milk supply,” Tracey Forfa, director of the FDA’s Center for Veterinary Medicine, told a webinar audience last week.
However, WHO’s Farrar has urged further caution by public health authorities “because it [the virus] may evolve into transmitting in different ways.”
“Do the milking structures of cows create aerosols? Is it the environment which they’re living in? Is it the transport system that is spreading this around the country?” he said.
“This is a huge concern, and I think we have to…make sure that if H5N1 did come across to humans with human-to-human transmission that we were in a position to immediately respond with access equitably to vaccines, therapeutics and diagnostics.”
According to a new European Food Safety Authority report, outbreaks of avian influenza continue to spread in the EU and beyond.
By Joshua Poole
To contact our editorial team please email us at
editorial@cnsmedia.com
If you found this article valuable, you may wish to receive our newsletters.
Subscribe now to receive the latest news directly into your inbox.
-



 Health23 hours ago
Health23 hours agoIt's possible to rely on plant proteins without sacrificing training gains, new studies say – The Globe and Mail
-
Business24 hours ago
Gildan replacing five directors ahead of AGM, will back two Browning West nominees – Yahoo Canada Finance
-



 Tech23 hours ago
Tech23 hours agoMeta Expands VR Operating System to Third-Party Hardware Makers – MacRumors
-



 Politics23 hours ago
Politics23 hours agoFareed’s take: There’s been an unprecedented wave of migration to the West – CNN
-



 Tech24 hours ago
Tech24 hours agoBrian's Randoms from Sea Otter 2024 – Pinkbike.com
-



 Science22 hours ago
Science22 hours agoNASA's Voyager 1 resumes sending engineering updates to Earth – Phys.org
-
Art16 hours ago
Made Right Here: Woodworking art – CTV News Kitchener
-
News22 hours ago
CTV National News: Honda's big move in Canada – CTV News






