Health
What you need to know about COVID-19 in Alberta on Wednesday, July 1 – CBC.ca
The latest:
- Large celebrations held yearly for Canada Day have been called off, but there are other activities — virtual and otherwise — still ongoing this July 1.
- Outdoor gathering limits were increased from 100 to 200 people on Tuesday for events like festivals, sporting events and performances, but not private gatherings like weddings.
- Alberta Safeway workers voted in favour of striking after the company ended its COVID-19 ‘hero pay’ program.
- Four outbreaks at Edmonton restaurants are responsible for 40 cases of COVID-19: Greta Bar (15 cases), Earl’s Tin Palace (6 cases), The Pint (10 cases) and Local (9 cases) on Jasper Avenue.
- An outbreak at a Calgary condo building, the Verve in East Village, has grown to 49 cases, 33 of which are active.
- Alberta will spend billions on infrastructure projects, cut its corporate tax rate, establish a new investment agency and introduce a series of targeted incentives for industry as part of a plan to restart its battered economy.
- A backlog of cattle waiting to be processed at meatpacking plants has put Alberta cattle ranchers in a tough position.
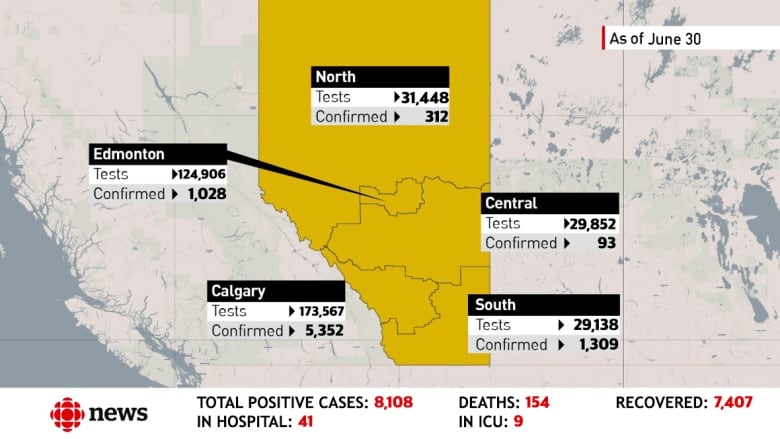
- The province reported 41 new cases of COVID-19 on Tuesday, for a total of 547 active cases.
- Two areas in Alberta are under “watch” as their active cases have surpassed a rate of 50 per 100,000 people: Calgary-Centre and Edmonton-Mill Woods West.
What you need to know today in Alberta:
Dr. Deena Hinshaw, the province’s chief medical officer of health, said the province has decided to increase its outdoor gathering limits, just in time for Canada Day, because it was seeing little community transmission of COVID-19 at public outdoor events.
Toronto’s city council has voted to make non-medical masks mandatory indoors, and Hinshaw said Alberta municipalities can look at their own circumstances and take appropriate measures.
Small to mid-sized businesses, co-operatives and non-profits impacted by COVID-19 were able to apply for grant relief on Monday morning — but some are saying that while the support is welcome, it won’t be enough on its own.
The Alberta government’s blueprint to reboot the economy was announced Monday in Calgary.
Alberta will increase spending on infrastructure projects, cut its corporate tax rate and establish a new investment agency as part of a plan to restart its battered economy.
Alberta reported 41 new COVID-19 cases on Tuesday.
In all, 154 people have died of COVID-19 in Alberta.


As of Tuesday, there are 547 active cases and 7,407 recovered in the province. The province has completed more than 449,000 tests for COVID-19.
Here’s how the active cases break down within provincial zones:
- Edmonton zone: 246 cases.
- Calgary zone: 232 cases.
- North zone: 36 cases.
- South zone: 28 cases.
- Central zone: 4 cases
- Unknown: 1 case.
What you need to know today in Canada:
Large-scale Canada Day events across the country have been cancelled, including the celebration and firewalks held yearly at Parliament Hill. Instead, a show was streamed live and will be followed by virtual fireworks.
Canada’s economy shrank by 11.6 per cent in April, the biggest plunge on record, following March’s contraction of 7.5 per cent as COVID-19 lockdowns began.
For the past few months, WestJet has barred the sale of adjacent seats throughout entire planes and Air Canada has followed suit in economy class. Those practices are set to end tomorrow.
As of 2:15 p.m. ET on Wednesday, Canada had 104,271 confirmed and presumptive coronavirus cases. Provinces and territories listed 67,746 of the cases as recovered or resolved. A CBC News tally of deaths based on provincial reports, regional health information and CBC’s reporting stood at 8,663. Wednesday’s tally did not include Ontario cases because of the Canada Day holiday.


Self-assessment and supports:
Alberta Health Services has an online self-assessment tool that you can use to determine if you have symptoms of COVID-19, but testing is open to anyone, even without symptoms.
The province says Albertans who have returned to Canada from other countries must self-isolate. Unless your situation is critical and requires a call to 911, Albertans are advised to call Health Link at 811 before visiting a physician, hospital or other health-care facility.
If you have symptoms, even mild, you are to self-isolate for 10 days from the onset of symptoms.
You can find Alberta Health Services’ latest coronavirus updates here.
The province also operates a confidential mental health support line at 1-877-303-2642 and addiction help line at 1-866-332-2322, available from 7 a.m. to 11 p.m., seven days a week.
Online resources are available for advice on handling stressful situations and ways to talk with children.
There is a 24-hour family violence information line at 310-1818 to get anonymous help in more than 170 languages, and Alberta’s One Line for Sexual Violence is available at 1-866-403-8000, from 9 a.m. to 9 p.m.
Health
April 22nd to 30th is Immunization Awareness Week – Oldies 107.7


<!–
isIE8 = true;
Date.now = Date.now || function() return +new Date; ;
Health
AHS confirms case of measles in Edmonton – CityNews Edmonton


Alberta Health Services (AHS) has confirmed a case of measles in Edmonton, and is advising the public that the individual was out in public while infectious.
Measles is an extremely contagious disease that is spread easily through the air, and can only be prevented through immunization.
AHS says individuals who were in the following locations during the specified dates and times, may have been exposed to measles.
- April 16
- Edmonton International Airport, international arrivals and baggage claim area — between 3:20 p.m. and 6 p.m.
- April 20
- Stollery Children’s Hospital Emergency Department — between 5 a.m. to 3 p.m.
- April 22
- 66th Medical Clinic (13635 66 St NW Edmonton) — between 12:15 p.m. to 3:30 p.m.
- Pharmacy 66 (13637 66 St NW Edmonton) — between 12:15 p.m. to 3:30 p.m.
- April 23
- Stollery Children’s Hospital Emergency Department — between 4:40 a.m. to 9:33 a.m.
AHS says anyone who attended those locations during those times is at risk of developing measles if they’ve not had two documented doses of measles-containing vaccine.
Those who have not had two doses, who are pregnant, under one year of age, or have a weakened immune system are at greatest risk of getting measles and should contact Health Link at 1-877-720-0707.
Symptoms
Symptoms of measles include a fever of 38.3° C or higher, cough, runny nose, and/or red eyes, a red blotchy rash that appears three to seven days after fever starts, beginning behind the ears and on the face and spreading down the body and then to the arms and legs.
If you have any of these symptoms stay home and call Health Link.
In Alberta, measles vaccine is offered, free of charge, through Alberta’s publicly funded immunization program. Children in Alberta typically receive their first dose of measles vaccine at 12 months of age, and their second dose at 18 months of age.
Health
U.S. tightens rules for dairy cows a day after bird flu virus fragments found in pasteurized milk samples – Toronto Star


/* OOVVUU Targeting */
const path = ‘/news/canada’;
const siteName = ‘thestar.com’;
let domain = ‘thestar.com’;
if (siteName === ‘thestar.com’)
domain = ‘thestar.com’;
else if (siteName === ‘niagarafallsreview.ca’)
domain = ‘niagara_falls_review’;
else if (siteName === ‘stcatharinesstandard.ca’)
domain = ‘st_catharines_standard’;
else if (siteName === ‘thepeterboroughexaminer.com’)
domain = ‘the_peterborough_examiner’;
else if (siteName === ‘therecord.com’)
domain = ‘the_record’;
else if (siteName === ‘thespec.com’)
domain = ‘the_spec’;
else if (siteName === ‘wellandtribune.ca’)
domain = ‘welland_tribune’;
else if (siteName === ‘bramptonguardian.com’)
domain = ‘brampton_guardian’;
else if (siteName === ‘caledonenterprise.com’)
domain = ‘caledon_enterprise’;
else if (siteName === ‘cambridgetimes.ca’)
domain = ‘cambridge_times’;
else if (siteName === ‘durhamregion.com’)
domain = ‘durham_region’;
else if (siteName === ‘guelphmercury.com’)
domain = ‘guelph_mercury’;
else if (siteName === ‘insidehalton.com’)
domain = ‘inside_halton’;
else if (siteName === ‘insideottawavalley.com’)
domain = ‘inside_ottawa_valley’;
else if (siteName === ‘mississauga.com’)
domain = ‘mississauga’;
else if (siteName === ‘muskokaregion.com’)
domain = ‘muskoka_region’;
else if (siteName === ‘newhamburgindependent.ca’)
domain = ‘new_hamburg_independent’;
else if (siteName === ‘niagarathisweek.com’)
domain = ‘niagara_this_week’;
else if (siteName === ‘northbaynipissing.com’)
domain = ‘north_bay_nipissing’;
else if (siteName === ‘northumberlandnews.com’)
domain = ‘northumberland_news’;
else if (siteName === ‘orangeville.com’)
domain = ‘orangeville’;
else if (siteName === ‘ourwindsor.ca’)
domain = ‘our_windsor’;
else if (siteName === ‘parrysound.com’)
domain = ‘parrysound’;
else if (siteName === ‘simcoe.com’)
domain = ‘simcoe’;
else if (siteName === ‘theifp.ca’)
domain = ‘the_ifp’;
else if (siteName === ‘waterloochronicle.ca’)
domain = ‘waterloo_chronicle’;
else if (siteName === ‘yorkregion.com’)
domain = ‘york_region’;
let sectionTag = ”;
try
if (domain === ‘thestar.com’ && path.indexOf(‘wires/’) = 0)
sectionTag = ‘/business’;
else if (path.indexOf(‘/autos’) >= 0)
sectionTag = ‘/autos’;
else if (path.indexOf(‘/entertainment’) >= 0)
sectionTag = ‘/entertainment’;
else if (path.indexOf(‘/life’) >= 0)
sectionTag = ‘/life’;
else if (path.indexOf(‘/news’) >= 0)
sectionTag = ‘/news’;
else if (path.indexOf(‘/politics’) >= 0)
sectionTag = ‘/politics’;
else if (path.indexOf(‘/sports’) >= 0)
sectionTag = ‘/sports’;
else if (path.indexOf(‘/opinion’) >= 0)
sectionTag = ‘/opinion’;
} catch (ex)
const descriptionUrl = ‘window.location.href’;
const vid = ‘mediainfo.reference_id’;
const cmsId = ‘2665777’;
let url = `https://pubads.g.doubleclick.net/gampad/ads?iu=/58580620/$domain/video/oovvuu$sectionTag&description_url=$descriptionUrl&vid=$vid&cmsid=$cmsId&tfcd=0&npa=0&sz=640×480&ad_rule=0&gdfp_req=1&output=vast&unviewed_position_start=1&env=vp&impl=s&correlator=`;
url = url.split(‘ ‘).join(”);
window.oovvuuReplacementAdServerURL = url;
Infected cows were already prohibited from being transported out of state, but that was based on the physical characteristics of the milk, which looks curdled when a cow is infected, or a cow has decreased lactation or low appetite, both symptoms of infection.
function buildUserSwitchAccountsForm()
var form = document.getElementById(‘user-local-logout-form-switch-accounts’);
if (form) return;
// build form with javascript since having a form element here breaks the payment modal.
var switchForm = document.createElement(‘form’);
switchForm.setAttribute(‘id’,’user-local-logout-form-switch-accounts’);
switchForm.setAttribute(‘method’,’post’);
switchForm.setAttribute(‘action’,’https://www.thestar.com/tncms/auth/logout/?return=https://www.thestar.com/users/login/?referer_url=https%3A%2F%2Fwww.thestar.com%2Fnews%2Fcanada%2Fu-s-tightens-rules-for-dairy-cows-a-day-after-bird-flu-virus-fragments-found%2Farticle_985b0bac-0252-11ef-abc6-eb884d6a1f0c.html’);
switchForm.setAttribute(‘style’,’display:none;’);
var refUrl = document.createElement(‘input’); //input element, text
refUrl.setAttribute(‘type’,’hidden’);
refUrl.setAttribute(‘name’,’referer_url’);
refUrl.setAttribute(‘value’,’https://www.thestar.com/news/canada/u-s-tightens-rules-for-dairy-cows-a-day-after-bird-flu-virus-fragments-found/article_985b0bac-0252-11ef-abc6-eb884d6a1f0c.html’);
var submit = document.createElement(‘input’);
submit.setAttribute(‘type’,’submit’);
submit.setAttribute(‘name’,’logout’);
submit.setAttribute(‘value’,’Logout’);
switchForm.appendChild(refUrl);
switchForm.appendChild(submit);
document.getElementsByTagName(‘body’)[0].appendChild(switchForm);
function handleUserSwitchAccounts()
window.sessionStorage.removeItem(‘bd-viafoura-oidc’); // clear viafoura JWT token
// logout user before sending them to login page via return url
document.getElementById(‘user-local-logout-form-switch-accounts’).submit();
return false;
buildUserSwitchAccountsForm();
console.log(‘=====> bRemoveLastParagraph: ‘,0);
-


 Science24 hours ago
Science24 hours agoNASA Celebrates As 1977’s Voyager 1 Phones Home At Last
-



 Politics24 hours ago
Politics24 hours agoPecker’s Trump Trial Testimony Is a Lesson in Power Politics
-
Media23 hours ago
B.C. puts online harms bill on hold after agreement with social media companies
-
Media17 hours ago
B.C. online harms bill on hold after deal with social media firms
-
Business23 hours ago
Oil Firms Doubtful Trans Mountain Pipeline Will Start Full Service by May 1st
-
Media22 hours ago
Trump poised to clinch US$1.3-billion social media company stock award
-
Real eState18 hours ago
Montreal tenant forced to pay his landlord’s taxes offers advice to other renters
-



 Politics17 hours ago
Politics17 hours agoPolitics Briefing: Younger demographics not swayed by federal budget benefits targeted at them, poll indicates




