Health
New map shows COVID-19 infections in Waterloo Region by neighbourhood – CTV News
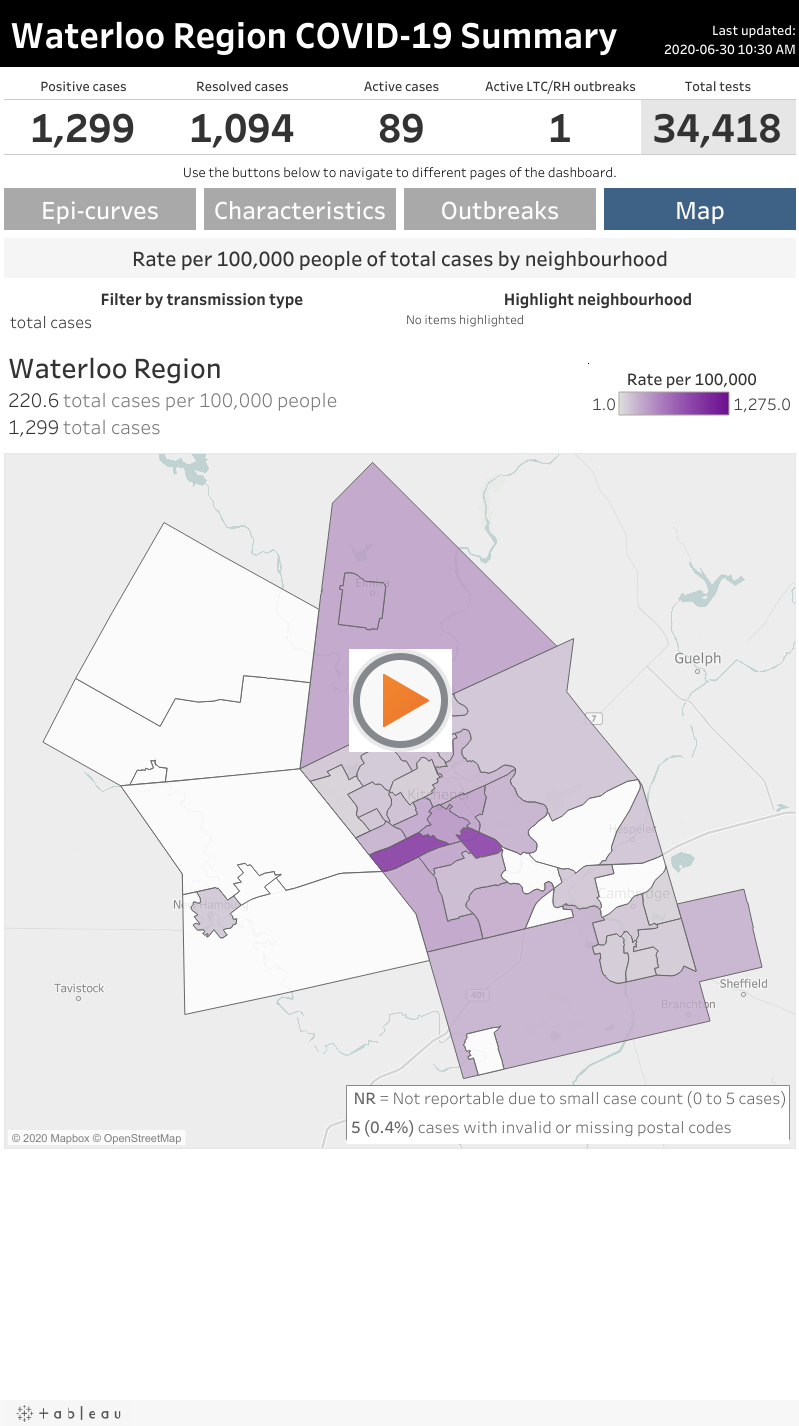
KITCHENER —
Region of Waterloo Public Health has launched a map that shows the highest concentration of infections in the region.
Accounting for both outbreak-related cases and non-outbreak cases, the Forest Heights/Forest Hill/Lakeside neighbourhood has the highest infection rate, at 992.9 per 100,000 people.
The Vanier/Rockway neighbourhood is next, with a rate of 945.6 cases per 100,000 people.
While both of those neighbourhoods have a high rate of outbreak-related cases, Vanier/Rockway also has the highest infection rate when adjusting for non-outbreak cases only.
Note: You can explore the map yourself by scrolling to the bottom of this article.
When looking at only non-outbreak cases, that neighbourhood has an infection rate of 260.2 per 100,000 people, with 41 cases.
Woolwich Rural North also has one of the highest rates of infection rate, with 212.9 infections per 100,000 people. There are 15 non-outbreak cases there.
The neighbourhood with the third-highest infection rate is Southwest Kitchener. There, the rate is 190.4 per 100,000 people.
Public health officials have reported 39 non-outbreak cases there, and 62 total cases.
On the region’s website, officials note that the data is skewed because of previous testing guidance that prioritized certain groups.
The website also notes that, just because your neighbourhood may look okay on the map, it doesn’t mean you’re safe.
“There is broad circulation of COVID-19 in our community. You should assume you could get COVID-19 anywhere in Waterloo Region,” the region’s website notes.
Several neighbourhoods on the map are considered not reportable because they have five cases or fewer.
The data also shows that five cases have invalid or missing postal codes.
Since the pandemic began, there have been 1,299 confirmed cases of COVID-19 in Waterloo Region. That includes 1,094 resolved cases and 116 deaths.
Having trouble viewing the map on the CTV News mobile app? You can see the interactive content on our mobile website.
Health
Avian influenza spread: WHO gives public health warning as FDA calms food safety concerns – Food Ingredients First


23 April 2024 — The World Health Organization (WHO) has warned that the ongoing spread of avian influenza poses a “significant public health concern” and urged health authorities, especially in the US, to closely monitor infections in cows. However, the US FDA maintains that the virus is not currently a concern to consumer health and downplayed its impact on commercial milk production.
Earlier this month, the largest producer of fresh eggs in the US halted production at a Texas plant after bird flu was detected in its chickens. Cal-Maine Foods said that about 3.6% of its total flock was destroyed after the infection.
However, the virus, also known as H5N1, has now been found in at least 26 dairy herds across eight US states, marking the first time this strain of bird flu has been detected in cattle, according to officials.
At least 21 states have restricted cattle importations from states where the virus is known to have infected dairy cows.
The US Department of Agriculture’s Animal and Plant Health Inspection Service strongly recommends minimizing the movement of cattle, but has not issued federal quarantine orders.
Public health threat
The US Centers for Disease Control and Prevention (CDC) confirmed this month that a dairy worker in Texas, who reportedly had exposure to dairy cattle presumed to have had avian influenza, contracted the virus and is now recovering.
“This infection does not change the H5N1 bird flu human health risk assessment for the US general public, which CDC considers to be low,” the agency said in a press release, while acknowledging that people who come into more frequent contact with possibly infected birds or other mammals have a higher risk.
Meanwhile, WHO’s chief scientist, Dr. Jeremy Farrar, told reporters recently in Geneva, Switzerland, that H5N1 has had an “extremely high” mortality rate among the several hundred people known to have been infected with it to date.


“H5N1 is an influenza infection, predominantly started in poultry and ducks and has spread effectively over the course of the last one or two years to become a global zoonotic — animal — pandemic,” said Farrar.
“The great concern, of course, is that in doing so and infecting ducks and chickens — but now increasingly mammals — the virus now evolves and develops the ability to infect humans.
“And then critically, the ability to go from human-to-human transmission.”
Concerns with cattle
US health officials have stressed that bird flu’s risk to the public is low, and the country’s food supply remains safe and stable.
“At this time, there continues to be no concern that this circumstance poses a risk to consumer health or that it affects the safety of the interstate commercial milk supply,” the FDA said in a statement.
According to officials, farmers are being urged to test cows that show symptoms of infection and separate them from the herd, where they usually recover within two weeks.
US producers are not permitted to sell milk from sick cows, while milk sold across state lines must be pasteurized or heat-treated to kill viruses, including influenza.

“We firmly believe that pasteurization provides a safe milk supply,” Tracey Forfa, director of the FDA’s Center for Veterinary Medicine, told a webinar audience last week.
However, WHO’s Farrar has urged further caution by public health authorities “because it [the virus] may evolve into transmitting in different ways.”
“Do the milking structures of cows create aerosols? Is it the environment which they’re living in? Is it the transport system that is spreading this around the country?” he said.
“This is a huge concern, and I think we have to…make sure that if H5N1 did come across to humans with human-to-human transmission that we were in a position to immediately respond with access equitably to vaccines, therapeutics and diagnostics.”
According to a new European Food Safety Authority report, outbreaks of avian influenza continue to spread in the EU and beyond.
By Joshua Poole
To contact our editorial team please email us at
editorial@cnsmedia.com
If you found this article valuable, you may wish to receive our newsletters.
Subscribe now to receive the latest news directly into your inbox.
Health
York Region urges you to get up to date on vaccinations – NewmarketToday.ca

York Region Public Health is reminding residents to keep up to date on their vaccinations as National Immunization Awareness Week begins.
The regional municipality said it is important to stay up to date on recommended vaccinations to ensure protection from contagious diseases. That includes updated COVID-19 vaccinations for vulnerable populations, recommended as part of a spring vaccination campaign.
“We know vaccines are safe and the best way to stay protected against vaccine-preventable disease,” the region said in a news release.
National Immunization Awareness Week runs from April 22 to 30, with this year’s theme being “Protect your future, get immunized.”
This spring, the region is still doing COVID-19 vaccinations. While walk-ins are no longer available as of April 2, you can book an appointment to visit a York Region clinic.
The spring COVID-19 vaccination campaign is aimed at more vulnerable groups who have received a COVID-19 vaccine before. Those include seniors, those living in seniors living facilities like long-term care homes, immunocompromised individuals and those in Indigenous households who are 55 or older. Public health also recommends the COVID-19 vaccine for those who have not yet received one.
York Region Public Health is also reminding residents of the need for other vaccines.
Measles cases have sprung up in Ontario and York Region recently. The region is recommending that people ensure they previously raised two valid doses of the measles vaccine. The region will also start providing measles vaccines April 29 for those overdue and for who do not have access to the vaccine through a health-care provider.
School-aged vaccinations are also available for free for children in junior kindergarten to Grade 12.
You can access immunization information at york.ca/immunziations or by contacting Access York at 1-877-464-9675.
“Vaccination helps protect everyone in our families, communities and schools,” the region said. “ By continuing to stay up to date on your immunizations, you help protect infants who are too young to be vaccinated and those not able to get vaccinated due to medical conditions.”
Health
Bird flu raises concern of WHO – ecns


The World Health Organization (WHO) said the rising number of bird flu cases has raised “great concern” because it had an “extremely high” mortality rate among those who had been infected around the world.
The WHO’s data show that from 2003 through March 2024, a total of 889 worldwide human cases of H5N1 infection had been recorded in 23 countries, resulting in 463 deaths and a 52 percent mortality rate. The majority of deaths occurred in Southeast Asian countries and Egypt.
The most recent death was in Vietnam in late March, when a 21-year-old male without underlying conditions died of the infection after bird hunting. So far, cases in Europe and the United States have been mild.
Jeremy Farrar, chief scientist at the WHO, said recently that H5N1, predominantly started in poultry and ducks, “has spread effectively over the course of the last one or two years to become a global zoonotic — animal — pandemic”.
He said that the great concern is that the virus is increasingly infecting mammals and then develops the ability to infect humans. It would become critical if the virus develops the ability to “go from human-to-human transmission”, Farrar said.
In the past month, health officials have detected H5N1 in cows and goats from 29 dairy herds across eight states in the US, saying it is an alarming development because those livestock weren’t considered susceptible to H5N1.
The development worries health experts and officials because humans regularly come into contact with livestock on farms. In the US, there are only two recorded cases of human infection — one in 2022 and one in April this year in Texas. Both infected individuals worked in close proximity to livestock, but their symptoms were mild.
Wenqing Zhang, head of the WHO’s global influenza program, told the Daily Mail that “bird-to-cow, cow-to-cow and cow-to-bird transmission have also been registered during these current outbreaks, which suggest that the virus may have found other routes of transition than we previously understood”.
Zhang said that multiple herds of cow infections in the US states meant “a further step of the virus spillover to mammals”.
The virus has been found in raw milk, but the Texas Health Services department has said the cattle infections don’t present a concern for the commercial milk supply, as dairies are required to destroy milk from sick cows. In addition, pasteurization also kills the virus.
Darin Detwiler, a former food safety adviser to the Food and Drug Administration and the US Agriculture Department, said that Americans should avoid rare meat and runny eggs while the outbreak in cattle is going on to avoid the possibility of infection from those foods.
Nevertheless, both the WHO and the Centers for Disease Control and Prevention (CDC) said that the risk the virus poses to the public is still low. Currently no human-to-human infection has been detected.
On the potential HN51 public health risk, Farrar cautioned that vaccine development was not “where we need to be”.
According to a report by Barron’s, under the current plan by the US Health and Human Services Department, if there is an H5N1 pandemic, the government would be able to supply a few hundred thousand doses within weeks, then 135 million within about four months.
People would need two doses of the shot to be fully protected. That means the US government would be able to inoculate about 68 million people — 20 percent — of 330 million in case of an outbreak.
The situation is being closely watched by scientists and health officials. Some experts said that a high mortality rate might not necessarily hold true in the event the virus became contagious among people.
“We may not see the level of mortality that we’re really concerned about,” Seema Lakdawala, a virologist at Emory University, told The New York Times. “Preexisting immunity to seasonal flu strains will provide some protection from severe disease.”
Agencies contributed to this story.
-



 Health21 hours ago
Health21 hours agoSee how chicken farmers are trying to stop the spread of bird flu – Fox 46 Charlotte
-



 Investment23 hours ago
Investment23 hours agoOwn a cottage or investment property? Here's how to navigate the new capital gains tax changes – The Globe and Mail
-



 Science20 hours ago
Science20 hours agoOsoyoos commuters invited to celebrate Earth Day with the Leg Day challenge – Oliver/Osoyoos News – Castanet.net
-
News20 hours ago
Freeland defends budget measures, as premiers push back on federal involvement – CBC News
-
News23 hours ago
‘A real letdown’: Disabled B.C. man reacts to federal disability benefit – Global News
-



 Politics20 hours ago
Politics20 hours agoHaberman on why David Pecker testifying is ‘fundamentally different’ – CNN
-
Economy20 hours ago
The Fed's Forecasting Method Looks Increasingly Outdated as Bernanke Pitches an Alternative – Bloomberg
-



 Science24 hours ago
Science24 hours agoEarly bird may dodge verticillium woes in potatoes – Manitobe Co-Operator









