Health
N.B. COVID-19 roundup: 7 new cases reported, suspected 4th case of variant detected – CBC.ca
Seven new cases of COVID-19 were reported in the province Friday, following news that a suspected fourth case of the coronavirus variant first reported in the U.K. has been detected in New Brunswick.
In an interview Friday morning, Premier Blaine Higgs said he doesn’t know where the variant case was located, but he was told a fourth one was found this week. This comes after the province announced Tuesday that it detected three variant cases.
“The cases that are known are isolating,” he said. “And they have had very limited contact with anyone.”
Higgs expects more cases of the variant will be detected, and he’s unsure how well it can be contained.
Chief Medical Officer of Health Dr. Jennifer Russell did not mention the suspected fourth case at Thursday’s COVID-19 news briefing. A Health Department spokesperson said the case is related to one of the first three variant cases.
“A close contact of a previous U.K. variant confirmed case has tested positive for COVID-19 and it is suspected they have the variant as well,” Bruce Macfarlane said in an emailed statement Friday morning.
He said a sample for sequencing is being sent to the National Microbiology Laboratory in Winnipeg for confirmation. In the meantime, the individual is being treated as if they have the variant.
Russell has said the arrival of the variant in New Brunswick is expected lead to larger outbreaks moving faster. It can become the dominant strain within three months. Age is the biggest risk factor.
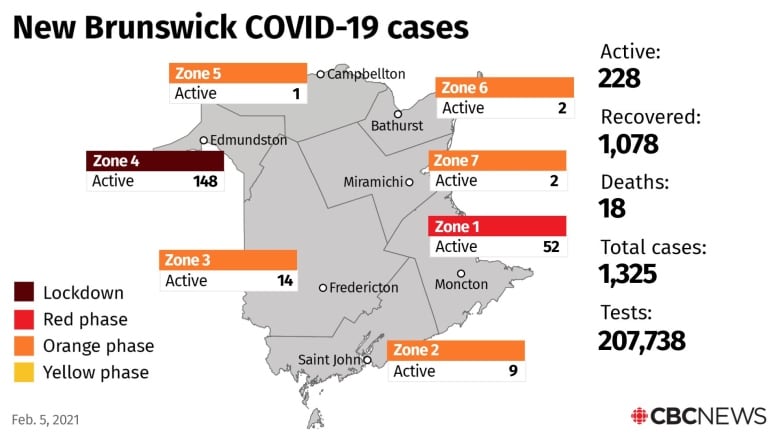

Seven new cases reported, in three zones
Public Health will not hold a live-streamed briefing Friday, but in a news release it said seven new cases have been recorded. The cases break down in this way:
Moncton region, Zone 1, one case:
- an individual 80-89
Fredericton region, Zone 3, one case:
- an individual 50-59
Edmundston region, Zone 4, five cases:
- an individual 20-29
- an individual 30-39
- three people 70-79
There are now 228 active cases in the province, and the number of confirmed cases so far in the pandemic is 1,325. Since Thursday, 35 people have recovered for a total of 1,078 recoveries.
There have been 18 deaths.
Six patients are in hospital, and three are in intensive care. A total of 207,738 tests have been conducted, including 1,819 since Thursday’s report.
New testing clinic opens in Campbellon
A new COVID-19 testing clinic has opened in the Campbellton Regional Hospital.
The new clinic will help with the increased demand in the region because of the mandatory weekly screening for travellers and people from border communities who are entering New Brunswick, Vitalite Health Network said in a news release Friday.
Tests are available by appointment only, by calling Tele-Care (811) or by filling out an online form on the gnb.ca website.
Vaccination of everyone could take until end of 2021
Premier Blaine Higgs says it could take until the end of 2021 for everyone in New Brunswick who wants a COVID-19 vaccine to get one.
But the premier still remains hopeful the province will see herd-immunity by September and that more vaccines will be approved before the end of the year.
“I have a degree of confidence,” he said during an interview with Information Morning Fredericton.
“Am I 100 per cent confident? No I’m not.”
Chief Medical Officer of Health Dr. Jennifer Russell says if New Brunswickers have a cold or flu symptom, it is important to get tested for COVID-19. 3:45
Higgs spoke with Prime Minister Justin Trudeau and other premiers at a meeting Thursday night where they discussed the vaccine’s availability.
Higgs said the premiers were told the country could expect to receive six million doses of the Moderna and Pfizer-BioNTech vaccines by the end of March.
But they were still left wondering about the exact timing of distribution, which Higgs says is concerning.
“We just want accuracy so we can communicate it,” he said.
Professor studies long-term effects of COVID-19
A biology professor at Mount Allison University is researching the long-term effects of COVID-19.
Vett Lloyd and her partners are asking people who have had COVID to take an online survey about their experiences.
The survey is available to everyone but is targeted at people from Ontario and New Brunswick.
Lloyd hopes the study will shed light on so called “long-haulers,” whose symptoms continue long after they contract the virus.
“It’s been coming out that some people, instead of fully recovering, remain ill to various extents . … So we’re trying to find out what their experiences are, what experiences or what it feels like to get COVID and then still be affected by it weeks, months later.”
More than 200 people have responded to the survey in its first few days.
Public exposure notification for Zone 3
Public Health has issued the following exposure notification for the Fredericton region, Zone 3:
- Carrington & Company, the gift shop at the Delta hotel at 225 Woodstock Rd., Fredericton, on Jan. 29 from 10 a.m. to 5 p.m. and on Feb. 1 from 11:30 a.m. to 5 p.m.
What to do if you have a symptom
People concerned they might have COVID-19 symptoms can take a self-assessment test online.
Public Health says symptoms shown by people with COVID-19 have included:
-
A fever above 38 C.
-
A new cough or worsening chronic cough.
-
Sore throat.
-
Runny nose.
-
Headache.
-
New onset of fatigue, muscle pain, diarrhea, loss of sense of taste or smell.
-
Difficulty breathing.
In children, symptoms have also included purple markings on the fingers and toes.
People with one of those symptoms should:
Health
AHS confirms case of measles in Edmonton – CityNews Edmonton

Alberta Health Services (AHS) has confirmed a case of measles in Edmonton, and is advising the public that the individual was out in public while infectious.
Measles is an extremely contagious disease that is spread easily through the air, and can only be prevented through immunization.
AHS says individuals who were in the following locations during the specified dates and times, may have been exposed to measles.
- April 16
- Edmonton International Airport, international arrivals and baggage claim area — between 3:20 p.m. and 6 p.m.
- April 20
- Stollery Children’s Hospital Emergency Department — between 5 a.m. to 3 p.m.
- April 22
- 66th Medical Clinic (13635 66 St NW Edmonton) — between 12:15 p.m. to 3:30 p.m.
- Pharmacy 66 (13637 66 St NW Edmonton) — between 12:15 p.m. to 3:30 p.m.
- April 23
- Stollery Children’s Hospital Emergency Department — between 4:40 a.m. to 9:33 a.m.
AHS says anyone who attended those locations during those times is at risk of developing measles if they’ve not had two documented doses of measles-containing vaccine.
Those who have not had two doses, who are pregnant, under one year of age, or have a weakened immune system are at greatest risk of getting measles and should contact Health Link at 1-877-720-0707.
Symptoms
Symptoms of measles include a fever of 38.3° C or higher, cough, runny nose, and/or red eyes, a red blotchy rash that appears three to seven days after fever starts, beginning behind the ears and on the face and spreading down the body and then to the arms and legs.
If you have any of these symptoms stay home and call Health Link.
In Alberta, measles vaccine is offered, free of charge, through Alberta’s publicly funded immunization program. Children in Alberta typically receive their first dose of measles vaccine at 12 months of age, and their second dose at 18 months of age.
Health
U.S. tightens rules for dairy cows a day after bird flu virus fragments found in pasteurized milk samples – Toronto Star


/* OOVVUU Targeting */
const path = ‘/news/canada’;
const siteName = ‘thestar.com’;
let domain = ‘thestar.com’;
if (siteName === ‘thestar.com’)
domain = ‘thestar.com’;
else if (siteName === ‘niagarafallsreview.ca’)
domain = ‘niagara_falls_review’;
else if (siteName === ‘stcatharinesstandard.ca’)
domain = ‘st_catharines_standard’;
else if (siteName === ‘thepeterboroughexaminer.com’)
domain = ‘the_peterborough_examiner’;
else if (siteName === ‘therecord.com’)
domain = ‘the_record’;
else if (siteName === ‘thespec.com’)
domain = ‘the_spec’;
else if (siteName === ‘wellandtribune.ca’)
domain = ‘welland_tribune’;
else if (siteName === ‘bramptonguardian.com’)
domain = ‘brampton_guardian’;
else if (siteName === ‘caledonenterprise.com’)
domain = ‘caledon_enterprise’;
else if (siteName === ‘cambridgetimes.ca’)
domain = ‘cambridge_times’;
else if (siteName === ‘durhamregion.com’)
domain = ‘durham_region’;
else if (siteName === ‘guelphmercury.com’)
domain = ‘guelph_mercury’;
else if (siteName === ‘insidehalton.com’)
domain = ‘inside_halton’;
else if (siteName === ‘insideottawavalley.com’)
domain = ‘inside_ottawa_valley’;
else if (siteName === ‘mississauga.com’)
domain = ‘mississauga’;
else if (siteName === ‘muskokaregion.com’)
domain = ‘muskoka_region’;
else if (siteName === ‘newhamburgindependent.ca’)
domain = ‘new_hamburg_independent’;
else if (siteName === ‘niagarathisweek.com’)
domain = ‘niagara_this_week’;
else if (siteName === ‘northbaynipissing.com’)
domain = ‘north_bay_nipissing’;
else if (siteName === ‘northumberlandnews.com’)
domain = ‘northumberland_news’;
else if (siteName === ‘orangeville.com’)
domain = ‘orangeville’;
else if (siteName === ‘ourwindsor.ca’)
domain = ‘our_windsor’;
else if (siteName === ‘parrysound.com’)
domain = ‘parrysound’;
else if (siteName === ‘simcoe.com’)
domain = ‘simcoe’;
else if (siteName === ‘theifp.ca’)
domain = ‘the_ifp’;
else if (siteName === ‘waterloochronicle.ca’)
domain = ‘waterloo_chronicle’;
else if (siteName === ‘yorkregion.com’)
domain = ‘york_region’;
let sectionTag = ”;
try
if (domain === ‘thestar.com’ && path.indexOf(‘wires/’) = 0)
sectionTag = ‘/business’;
else if (path.indexOf(‘/autos’) >= 0)
sectionTag = ‘/autos’;
else if (path.indexOf(‘/entertainment’) >= 0)
sectionTag = ‘/entertainment’;
else if (path.indexOf(‘/life’) >= 0)
sectionTag = ‘/life’;
else if (path.indexOf(‘/news’) >= 0)
sectionTag = ‘/news’;
else if (path.indexOf(‘/politics’) >= 0)
sectionTag = ‘/politics’;
else if (path.indexOf(‘/sports’) >= 0)
sectionTag = ‘/sports’;
else if (path.indexOf(‘/opinion’) >= 0)
sectionTag = ‘/opinion’;
} catch (ex)
const descriptionUrl = ‘window.location.href’;
const vid = ‘mediainfo.reference_id’;
const cmsId = ‘2665777’;
let url = `https://pubads.g.doubleclick.net/gampad/ads?iu=/58580620/$domain/video/oovvuu$sectionTag&description_url=$descriptionUrl&vid=$vid&cmsid=$cmsId&tfcd=0&npa=0&sz=640×480&ad_rule=0&gdfp_req=1&output=vast&unviewed_position_start=1&env=vp&impl=s&correlator=`;
url = url.split(‘ ‘).join(”);
window.oovvuuReplacementAdServerURL = url;
Infected cows were already prohibited from being transported out of state, but that was based on the physical characteristics of the milk, which looks curdled when a cow is infected, or a cow has decreased lactation or low appetite, both symptoms of infection.
function buildUserSwitchAccountsForm()
var form = document.getElementById(‘user-local-logout-form-switch-accounts’);
if (form) return;
// build form with javascript since having a form element here breaks the payment modal.
var switchForm = document.createElement(‘form’);
switchForm.setAttribute(‘id’,’user-local-logout-form-switch-accounts’);
switchForm.setAttribute(‘method’,’post’);
switchForm.setAttribute(‘action’,’https://www.thestar.com/tncms/auth/logout/?return=https://www.thestar.com/users/login/?referer_url=https%3A%2F%2Fwww.thestar.com%2Fnews%2Fcanada%2Fu-s-tightens-rules-for-dairy-cows-a-day-after-bird-flu-virus-fragments-found%2Farticle_985b0bac-0252-11ef-abc6-eb884d6a1f0c.html’);
switchForm.setAttribute(‘style’,’display:none;’);
var refUrl = document.createElement(‘input’); //input element, text
refUrl.setAttribute(‘type’,’hidden’);
refUrl.setAttribute(‘name’,’referer_url’);
refUrl.setAttribute(‘value’,’https://www.thestar.com/news/canada/u-s-tightens-rules-for-dairy-cows-a-day-after-bird-flu-virus-fragments-found/article_985b0bac-0252-11ef-abc6-eb884d6a1f0c.html’);
var submit = document.createElement(‘input’);
submit.setAttribute(‘type’,’submit’);
submit.setAttribute(‘name’,’logout’);
submit.setAttribute(‘value’,’Logout’);
switchForm.appendChild(refUrl);
switchForm.appendChild(submit);
document.getElementsByTagName(‘body’)[0].appendChild(switchForm);
function handleUserSwitchAccounts()
window.sessionStorage.removeItem(‘bd-viafoura-oidc’); // clear viafoura JWT token
// logout user before sending them to login page via return url
document.getElementById(‘user-local-logout-form-switch-accounts’).submit();
return false;
buildUserSwitchAccountsForm();
console.log(‘=====> bRemoveLastParagraph: ‘,0);
Health
New technology to advance women’s cancer care at Southlake


|
|
NEWS RELEASE
SOUTHLAKE REGIONAL HEALTH CENTRE
**************************
This Cancer Awareness Month, Southlake is adding advanced technologies to detect and treat breast cancer and other women’s cancers thanks to generous community donor support, most recently through the HERE is Where Cancer Meets its Match campaign. New cancer care technology, including new mammography machines, the MyoSure System and the MOLLI 2® System will make a measurable impact in diagnosing and treating women’s cancers in the communities Southlake serves.
Southlake is installing three new mammography machines to expand its breast cancer screening program to 1,500 more women each year. Two of these machines have new biopsy capabilities that will reduce the number of cancelled exams due to equipment failure, ensuring timely care for women. Women ages 40 to 49 years old will be able to self-refer for publicly funded mammograms through the Ontario Breast Screening Program starting this fall.
“Early detection is critical when treating breast cancer and other women’s cancers,” said Lorrie Reynolds, Director, Regional Cancer Program at Southlake. “We treat more than 1,700 breast cancer patients at Southlake every year. By adding advanced technology, like the new mammography machines, we’re ensuring women have the best experience at Southlake.”
Southlake is also introducing the MyoSure System, an innovative technology that can help detect female reproductive cancers. Damaged tissue in a woman’s uterus such as fibroids and polyps can now be removed in a precise, minimally invasive procedure that leaves the rest of the uterus intact. This will improve the overall patient experience by supporting faster recovery, reducing the risk of infection and giving more women the option to have children. An estimated 200 women per year will benefit from the MyoSure System.
The new mammography machines and the MyoSure System build on Southlake’s recent investment in the MOLLI 2® System, a made-in-Canada wire-free breast localization technology. This technology is considerably less invasive and more accurate when compared to wire-guided localization, resulting in a better patient experience and improved cosmetic outcomes. More than 200 women each year will benefit from this innovative medical device as they are treated for breast cancer at Southlake.
“As a clinician caring for women with cancer in our community, I’m incredibly proud of the work Southlake is doing to advance women’s health and improve patient experiences,” said Sara Temple, MD, Surgical Oncologist and Chief of Surgery at Southlake. “Women who visit Southlake can be confident that they are receiving leading edge care, close to home when they need it most.”
The World Health Organization anticipates a 77 per cent increase in cancer diagnoses by 2050. Southlake serves some of the fastest growing communities in Canada and anticipates that the number of patients requiring cancer care will grow. By investing in new technology, Southlake is ensuring that women in the communities it serves have access to leading edge cancer care. All of these investments were funded with support from community donors who generously gave to Southlake to support investments into women’s health at the hospital.
“The generosity of our donor community and the impact they have made for women receiving cancer diagnosis and treatment at Southlake is something we can all take great pride in,” said Jennifer Ritter, President and CEO of Southlake Foundation. “From our Women’s Health Initiative donors supporting new mammography machines, to the Ladies in Philanthropy for Southlake funding the MOLLI 2 System, to our long-standing partners The Edge Benefits and Pheasant Run Golf Club enabling the introduction of MyoSure System through their joint annual charity golf tournament, we are incredibly lucky to share a vision of access to exceptional care for everyone who depends on Southlake when they need us most. Thank you, to every donor who contributed to these important upgrades to care for women.”
Southlake Foundation’s HERE is Where Cancer Meets its Match campaign supports the Stronach Regional Cancer Centre at Southlake. For more information or to make a donation, visit: southlake.ca/HERE.





-
News21 hours ago
Amid concerns over ‘collateral damage’ Trudeau, Freeland defend capital gains tax change
-
Art19 hours ago
The unmissable events taking place during London’s Digital Art Week
-



 Politics23 hours ago
Politics23 hours agoHow Michael Cohen and Trump went from friends to foes
-



 Real eState24 hours ago
Real eState24 hours agoBlending Function and Style: The Best Garage Door Designs for Contemporary Homes
-
News22 hours ago
U.K. tabloids abuzz with Canadian’s ‘Loch Ness monster’ photo
-
Health24 hours ago
Interior Health delivers nearly 800K immunization doses in 2023
-



 Politics22 hours ago
Politics22 hours agoPolitics Briefing: Saskatchewan residents to get carbon rebates despite province’s opposition to pricing program
-
News20 hours ago
What is a halal mortgage? How interest-free home financing works in Canada







